Chikungunya Vaccine Test Launched in Puerto Rico

A promising prophylactic vaccine candidate against the chikungunya fever will be tested in a phase II clinical study in Puerto Rico.
Puerto Rico is a chikungunya fever endemic area.
For the development of this live attenuated Chikungunya vaccine, genes coding for selected antigens from the Chikungunya virus have been inserted into the genome of the well-established measles vaccine.
Measles vaccines have been successfully used in hundreds of millions of people globally, which means it offers an excellent safety profile.
Those new antigens are thus produced within the cells, thereby triggering a specific immune response against the Chikungunya virus.
According to the vaccine manufacturer, the vaccine candidate already showed high seroconversion rates in the preceding phase I clinical trial. Up to 100 percent of all vaccinated subjects produced neutralizing antibodies against the chikungunya virus.
The additional trial in Puerto Rico is intended to evaluate the influence of previous chikungunya infections on the safety and immunogenicity of the vaccine candidate.
There is no specific antiviral therapy for chikungunya virus infection.
Commenting on the trial, Dr. Erich Tauber, CEO and co-founder of the biotech company Themis Bioscience GmbH, says, "This study will complement our two ongoing Phase II studies with additional data that are of specific importance for the safety of the vaccine in areas where Chikungunya fever is or has been endemic," said Dr. Tauber.
The term Chikungunya means “to be contorted” in the Kimakonde language, describing the crouched physical appearance of anguished patients.
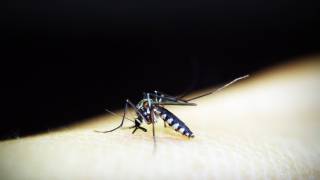
Chikungunya virus is primarily transmitted to humans through the bites of infected mosquitoes, predominantly Aedes aegypti and Aedes albopictus. Within the last four years, the chikungunya virus has sickened 2,527,280 people from 60 countries in Europe, the Americas, Asia and Africa, according to Pan American Health Organization (PAHO) data.
As of late June 2017, there have been 140,569 chikungunya cases reported in the Americas.
Outbreaks of dengue, Zika, and chikungunya viruses are taking place in several countries. The simultaneous spreading of these arboviruses raises the possibility of co-infections in people. Indeed, several cases of dengue and chikungunya co-infections have already been reported by the PAHO.
The dengue virus has had epidemic status in Brazil since the 1980s, while the chikungunya virus was only identified in Brazil in 2014. Zika virus was first discovered in 1947 in the Zika Forest of Uganda during an intensive search for yellow fever virus.
The majority of people infected with chikungunya virus become symptomatic.
The incubation period is typically 3–7 days, with acute symptoms typically resolved within 7–10 days.
The disease is most often characterized by acute onset of fever and polyarthralgia. Joint symptoms can be severe and debilitating. Other symptoms may include headache, myalgia, arthritis, conjunctivitis, nausea/vomiting, or maculopapular rash.
Themis Bioscience GmbH develops prophylactic vaccines from the preclinical to the early clinical phase, focusing on emerging tropical infectious diseases, with initial vaccine candidates currently being developed against Chikungunya and Zika.
Based in Vienna, Themis Bioscience’s virus vaccine vector technology is licensed from the Institut Pasteur in Paris. Its Chikungunya vaccine Phase I/II trial in the US is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Our Trust Standards: Medical Advisory Committee