Cholera Vaccines
Cholera Vaccines 2024
The U.S. Food and Drug Administration (FDA), the European Medicine Agency (EMA), and the U.K. NHS recommend oral cholera vaccines (OCV) for specific conditions in countries that are undergoing outbreaks in 2023. Vaccination against cholera is not generally recommended because most international travelers do not visit cholera outbreaks. In August 2023, the U.S. Centers for Disease Control and Prevention (CDC) published Cholera Vaccine: Recommendations, highlighting CVD 103-HgR (Vaxchora®) for travelers ages 2–64 years old going to areas of active toxigenic Vibrio cholerae O1 transmission. As of 2024, the World Health Organization (WHO) has prequalified Dukoral®, Shanchol™, and Euvichol® OCVs. On April 17, 2024, the WHO prequalified Euvichol-S.
Cholera Vaccines
All OCVs require two vaccine doses for complete protection against cholera for up to three years, while a single dose provides short-term protection, says the WHO.
Dukoral® is administered with a buffer solution that requires 150 ml of clean water for adults. It can be given to all individuals over the age of 2.
Shanchol™ and Euvichol® are essentially the same vaccines produced by two different manufacturers.
Euvichol-Plus® is an inactivated OCV jointly developed by Eubiologics and the International Vaccine Institute for cholera prevention. Euvichol®-S improves productivity by approximately 40% over Euvichol-Plus®.
Vaxchora® (lyophilized CVD 103-HgR) is a single-dose, oral vaccine, U.S. FDA-approved in June 2016. The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons
Cholera Vaccine Availability 2024
The International Coordinating Group (ICG) for OCVs was created in 1997 to manage the global stockpile. Since its establishment, the WHO, UNICEF, and Médecins sans Frontières have facilitated about 73 million doses of OCV for 23 countries. As of April 2024, the WHO stated the ongoing global cholera response has been affected by a critical shortage of OCVs. Global production capacity in 2024 is forecast to be 37-50 million doses. From January 2023 to January 2024, 76 million OCV doses were requested by 14 countries, while only 38 million doses were available during that period, according to the WHO External Situation Report Edition 13. GAVI says that in 2024, approximately 50 million OCV doses are forecasted to be available to the global stockpile this year, compared to 38 million in 2023. As of April 2024, South Korea-based EuBiologics is the only OCV supplier to the global stockpile.
The worldwide supply of OCV in 2022 was 36 million doses. Gavi, the Vaccine Alliance, published a roadmap on May 22, 2023, that outlines actions needed to ensure the supply of OCV can meet growing international demand by 2026. GAVI says in the current outbreak context, only one OCV dose course has been validated and implemented in reactive vaccination campaigns.
Cholera Outbreaks
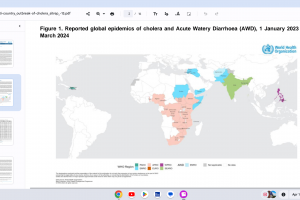
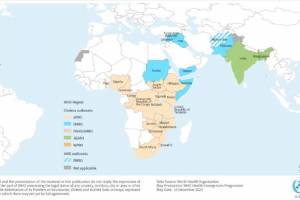
Cholera outbreaks continued in 2024.
Cholera Vaccine News 2024
April 15, 2024 - EuBiologics and IVI announced that Euvichol-S achieved WHO prequalification.
March 20, 2024 - The WHO stated: We appeal to vaccine manufacturers, governments, donors, and partners to prioritize an urgent scale-up of vaccine production and invest in all the efforts needed to prevent and control cholera.
February 12, 2024 - The WHO published situation report #11.
December 7, 2023 - The WHO published report #9 regarding active cholera outbreaks.
November 2, 2023 - The WHO published a Multi-country cholera outbreak, External Situation Report #8.
August 28, 2023 - The Republic of Kenya vaccinated about 1.6 million people with OCVs in August 2023.
June 1, 2023 - The WHO published a Multi-country cholera outbreak, External Situation Report #3.
May 15, 2023 - The WHO published a Multi-country cholera outbreak, External Situation Report #2.
March 22, 2023 - The WHO published - Multi-country Cholera Outbreaks, External Situation Report #1.
December 5, 2022 - The U.S. CDC published Travelers Returning to the U.S. with Cholera – Information and CDC Guidance for Healthcare Providers.
October 19, 2022—The WHO Director-General stated that four agencies decided to suspend the two-dose OCV vaccination strategy in favor of a one-dose approach so that more people receive some protection from limited stocks.