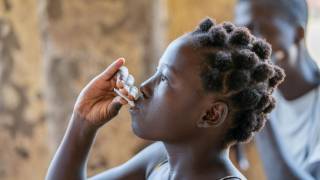Simplified Oral Cholera Vaccine Prequalified by the WHO

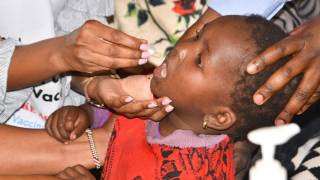
For many years, oral vaccines have proven to be the quickest intervention for preventing, limiting, and controlling cholera outbreaks.
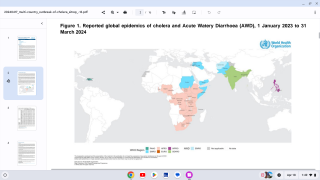
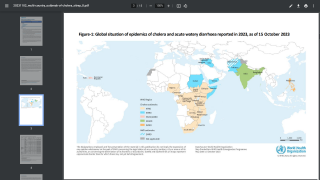
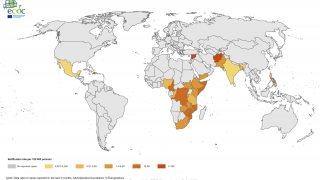
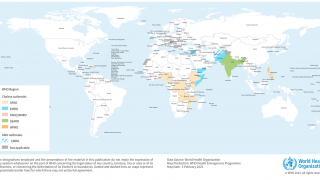
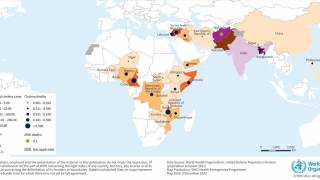
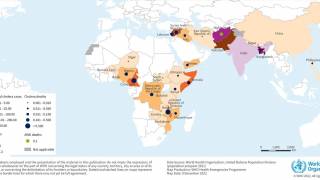
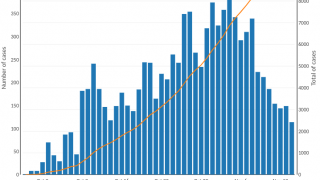
However, the supply of these vaccines was at an all-time low in 2024, especially in 23 countries, including Comoros, the Democratic Republic of the Congo, Ethiopia, Mozambique, Somalia, Zambia, and Zimbabwe.
To help relieve this inventory shortage, the World Health Organization (WHO) issued a new oral cholera vaccine (OCV) prequalification.
On April 12, 2024, EuBiologicals Co., Ltd.'s inactivated oral vaccine, Euvichol-S, which has similar efficacy to existing vaccines but a simplified formulation, was announced.
This authorization creates new opportunities to increase OCV production capacity rapidly.
"The new vaccine is the third product of the same family of cholera vaccines on our WHO prequalification list," said Dr Rogerio Gaspar, Director of the WHO Department for Regulation and Prequalification, in a press release on April 18, 2024.
The WHO's OCV prequalification list already includes Euvichol, Euvichol-Plus, Vaxchora®, Dukoral®, and Shanchol™.
When visiting countries with cholera outbreaks in 2024, the U.S. Centers for Disease Control and Prevention recommends OCV vaccination one month before traveling.
Our Trust Standards: Medical Advisory Committee