Another Dengue Vaccine Candidate Found Very Effective

An Original Article by the New England Journal of Medicine today stated that in 2024, there is an unmet global need for a dengue vaccine that offers protection from all four virus types in a single dose across a wide age range, regardless of dengue serostatus at baseline.
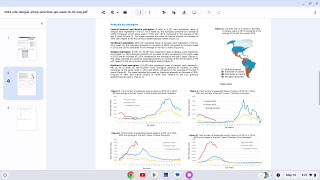
Published on February 1, 2024, this article concluded a phase 3 clinical trial found a single dose of Butantan–Dengue Vaccine (Butantan-DV) prevented symptomatic DENV-1 and DENV-2, regardless of dengue serostatus at baseline, through 2 years of follow-up.
Over the 2-year follow-up, vaccine efficacy against any DENV serotype was 79.6% (95% CI, 70.0 to 86.3).
Secondary endpoints of serotype-specific vaccine efficacy were 89.5% (95% CI, 78.7 to 95.0) against DENV-1 and 69.6% (95% CI, 50.8 to 81.5) against DENV-2.
Regarding baseline dengue serostatus, vaccine efficacy against any serotype was 73.6% (95% CI, 57.6 to 83.7) among participants without evidence of previous dengue exposure (7516 participants) and 89.2% (95% CI, 77.6 to 95.6) among those with evidence of prior dengue exposure (8017 participants).
Butantan-DV is a live, attenuated, tetravalent dengue vaccine candidate composed of vaccine viruses representing all four DENV serotypes analogous to the TV003 formulation developed by the U.S. National Institutes of Health.
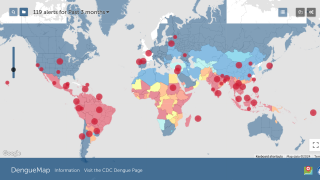
Currently, two dengue vaccines are being used by various countries confronted by outbreaks.
Our Trust Standards: Medical Advisory Committee