Hexavalent VLP Norovirus Vaccine Candidate License Agreement Announced
HilleVax, Inc. and Chengdu Kanghua Biological Products Co., Ltd. today announced the entry into an exclusive license agreement for rights to Kangh's hexavalent virus-like particle (VLP) vaccine candidate for norovirus.
Referred by HilleVax as HIL-216, outside of Greater China, this VLP targets six of the most common norovirus genotypes, including GI.1, GII.2, GII.3, GII.4, GII.6, and GII.17.
HilleVax stated it intends to launch a Phase 1 trial in 2024.
According to the press release on January 8, 2024, the Investigational New Drug application for HIL-216 was cleared by the U.S. FDA in September 2023.
As of January 9, 2024, the FDA has not approved any norovirus vaccine candidate for use in the U.S.
Rob Hershberg, MD, Ph.D., Chairman and Chief Executive Officer at Hillevax, commented, "Our bivalent norovirus VLP vaccine candidate, HIL-214, remains the most advanced norovirus vaccine candidate in clinical development, and we are on track to report topline safety and efficacy data in mid-2024."
"We believe that HIL-214 will be the first norovirus vaccine submitted for registration and, if approved, would address the significant unmet medical need in infants and other at-risk populations."
"We further believe that HIL-216 is an exciting addition to the HilleVax portfolio as a next-generation, higher valency VLP-based vaccine and is an ideal fit with the expertise, capabilities, and long-term aspirations of HilleVax."
HilleVax confirmed it will pay Kangh an upfront payment of $15 million with the potential for additional payments of up to $255.5 million upon achieving specific development and sales milestones. Kangh can also receive a single-digit tiered royalty on net sales outside of Greater China.
Globally, norovirus is estimated to result in approximately 700 million cases of acute gastroenteritis and 200,000 deaths per year, resulting in over $4 billion in direct health system costs and $60 billion in societal costs per year.
According to the U.S. CDC, norovirus is a contagious virus that causes vomiting and diarrhea. Anyone can get infected and sick with norovirus. Most norovirus outbreaks in the U.S. happen from November to April.
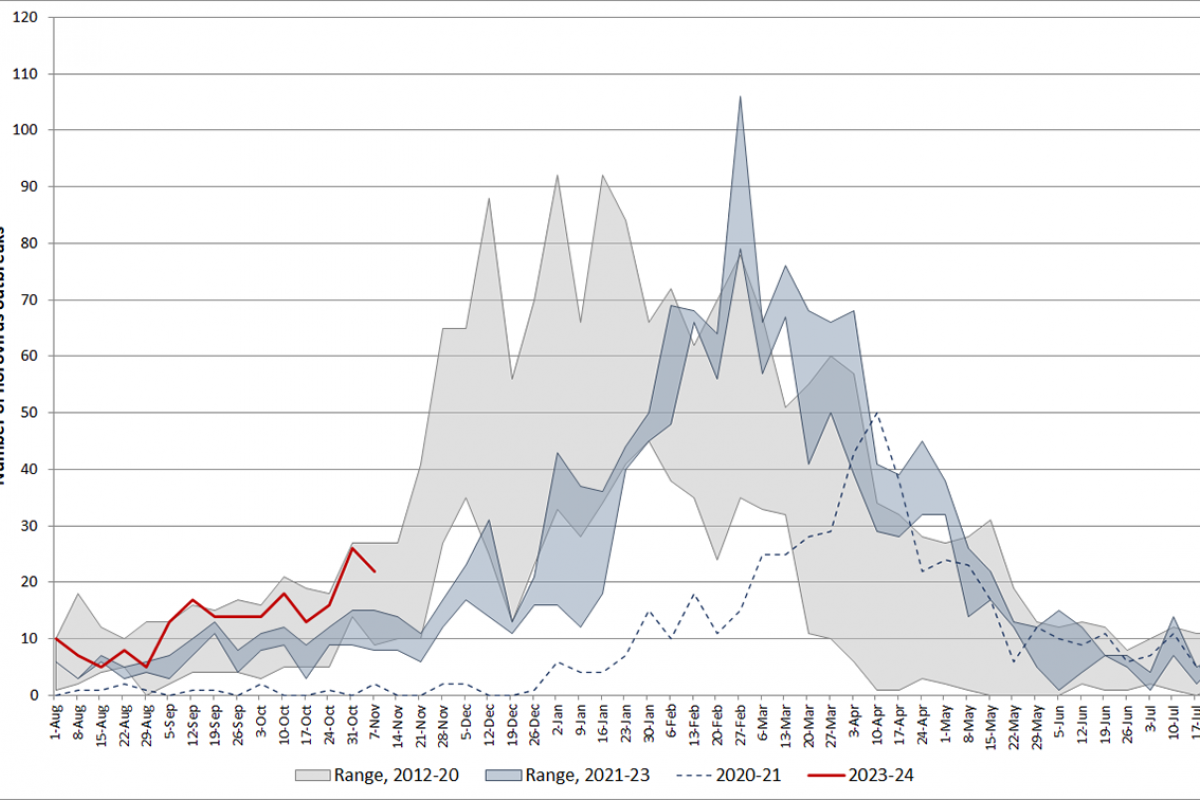
From August through November 13, 2023, there were 202 norovirus outbreaks reported by NoroSTAT-participating states. During the same period last season, 134 norovirus outbreaks were reported by these states, according to the CDC.
HilleVax is a clinical-stage biopharmaceutical company based in Boston, MA, focused on developing and commercializing novel vaccines.
Our Trust Standards: Medical Advisory Committee