Lactating Mothers Included in Norovirus Vaccine Candidate Study

Vaxart, Inc. today announced that it has completed enrollment and dosing in the Phase 1 clinical trial evaluating Vaxart’s oral pill bivalent norovirus vaccine candidate focused on lactating mothers.
There is no U.S. Food and Drug Administration-approved vaccine against norovirus.
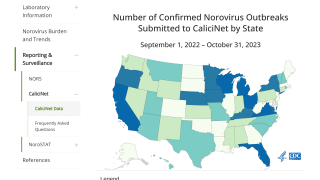
Outbreaks sickens approximately 21 million people in the United States annually, and 15% of children under age five contract norovirus annually.
Approximately 3 million sets of parents are forced by this virus to miss work, 2.2 days on average, to care for their children. The annual disease burden from norovirus is $10.6 billion in the U.S. alone.
“This is an important step forward as we drive toward a vaccine candidate that may make it possible for mothers to protect their children against this highly contagious – and potentially lethal -- virus. We look forward to announcing topline data from this study by the end of 2024,” commented Dr. James F. Cummings, Vaxart’s Chief Medical Officer, in a press release on December 21, 2023.
“We are very proud of our clinical team for completing enrollment of this trial within our planned timeline.”
The Phase 1, multicenter, randomized, double-blind, placebo-controlled single dose, dose-ranging study (VXA-NVV-108) is designed to evaluate the safety, tolerability, and immunogenicity of orally administered bivalent GI.1/GII.4 norovirus vaccine in healthy lactating females of at least 18 years of age.
As of late December 2023, several norovirus vaccine candidates are conducting clinical research.
Our Trust Standards: Medical Advisory Committee