Ebola Vaccine Found Safe For Infants
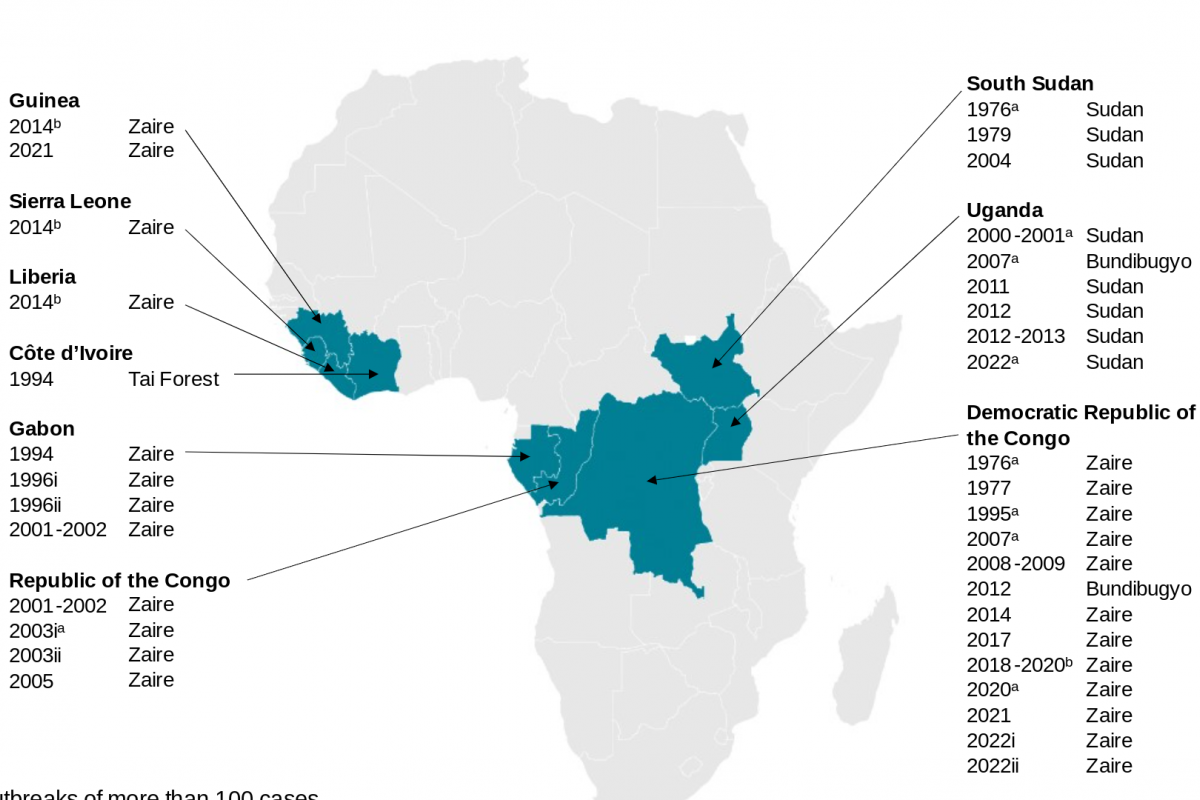
Since the discovery of the Ebola virus disease (EVD) in 1976, more than 30 outbreaks have been reported in Africa. While Ebola vaccines have been approved for adults, infants have not been protected from the EVD.
Published by The Lancet Global Health in November 2023, this analysis concluded an Ebola vaccine combination was well tolerated and induced strong humoral responses in infants younger than one year.
Furthermore, this phase 2 study concluded there were no safety concerns related to Zabdeno® (Ad26.ZEBOV) and Mvabea® (MVA-BN-Filo) vaccination.
And the reactogenicity profile comprised mild-to-moderate adverse events (grade 1 or 2). Within seven days of administration of either dose, there were no grade 3 solicited adverse events in the Ebola vaccine group.
The two-dose Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen induced robust antibody responses in 100% of infants 21 days after receiving dose two.
The serum antibody levels declined over the follow-up period, but 93% of the younger and 100% of the older infants were still considered responders 12 months post-dose 1.
In addition to its EMA-approved use in individuals aged one year or older, the results of the current Vaccines & Prevention B.V.-funded study could support the use of Ad26.ZEBOV and MVA-BN-Filo in infants aged 4–11 months, as recommended for off-label use in 2021.
As of November 30, 2023, access to Ebola vaccinations in the U.S. is limited. The U.S. CDC updated its Ebola Overbreak History on August 30, 2023.
Our Trust Standards: Medical Advisory Committee























