New Zika Vaccine Candidate Launches Clinical Study
The first participant recently received a dose of a Zika virus vaccine candidate in a phase 1 clinical trial conducted by the University of Liverpool at the Clinical Research Facility within the Royal Liverpool University Hospital.
This study is essential since Zika remains an ongoing threat, with thousands of cases of the mosquito-borne virus reported annually, in countries such as Brazil, Belize, Guatemala, Paraguay, and Bolivia.
And in 2023, Puerto Rico reported 21 probable Zika cases.
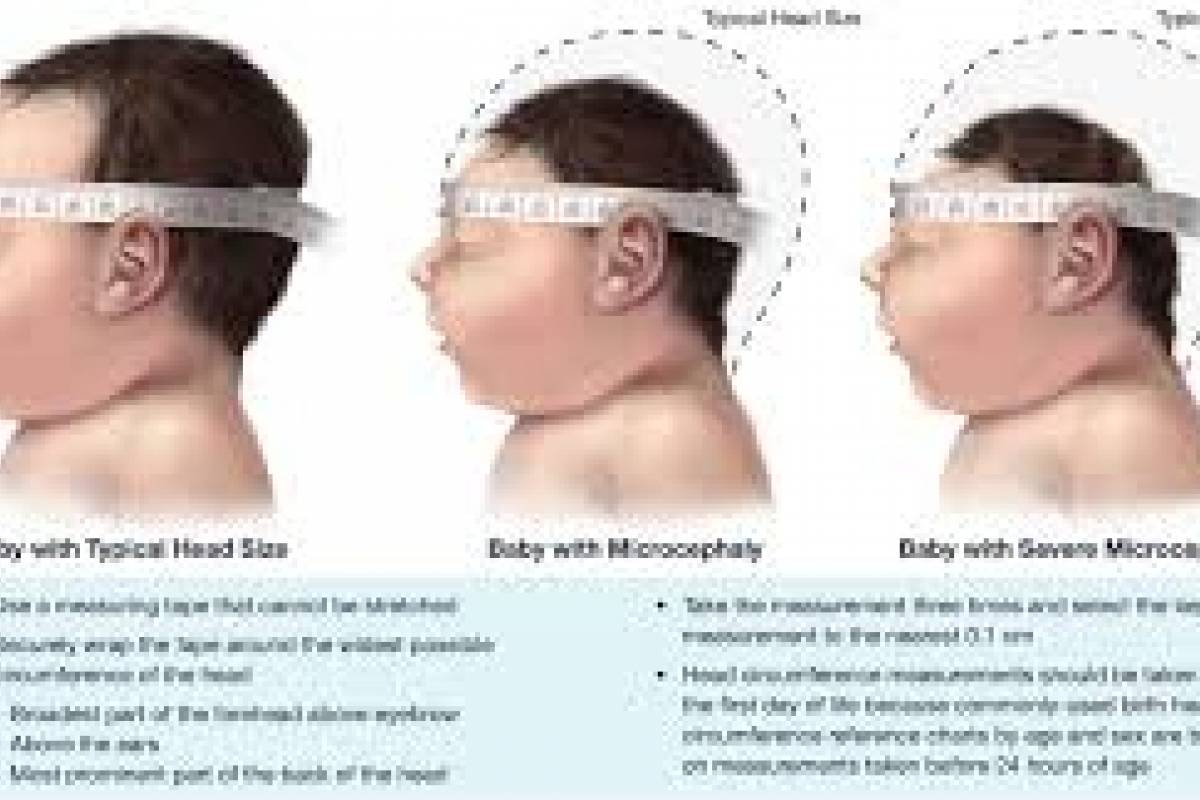
And pregnant women continue at the highest risk for the infection as the virus can cause severe fetal congenital disabilities, such as microcephaly, a condition where a baby's head is much smaller than expected.
It's hoped that the vaccine, designed to be suitable for use during pregnancy, wrote these researchers on May 2, 2023.
“Zika should not be forgotten, especially since climate change contributes to the spread of the Aedes mosquitoes to countries without immunity. Vaccines like ours will enable us to be better prepared for the next Zika outbreak,” commented Dr. Krishanthi Subramaniam in a press release on April 27, 2023.
The vaccine originates from a 2016 Zika Rapid Response grant awarded to Dr. Tom Blanchard and colleagues at the University of Manchester in collaboration with the UK Health Security Agency.
Dr, Blanchard has since developed several iterations to enhance the vaccine's effectiveness and manufacturing scale-up.
Liverpool researchers have been driving this project forward since 2017. The team has used an approach to develop a vaccine based on studies to understand immunity to Zika and other related viruses.
As of May 11, 2023, there are no U.S. FDA-approved Zika vaccines.
Our Trust Standards: Medical Advisory Committee