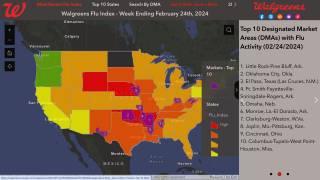
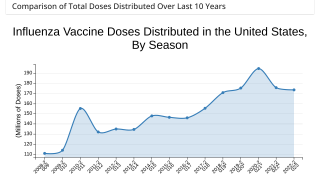
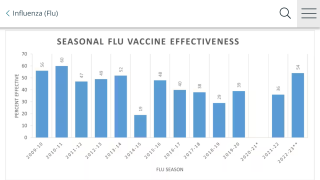
Influenza and COVID-19 Combo Vaccine Candidate Posts Positive Phase 3 Data

Moderna, Inc. today announced that its Phase 3 clinical trial of mRNA-1083, an investigational combination vaccine against influenza and COVID-19, has met its primary endpoints, eliciting a higher immune response than the licensed comparator vaccines used in the trial.
mRNA-1083 met its primary endpoints, eliciting higher immune responses against influenza and SARS-CoV-2 viruses than licensed flu and COVID vaccines in adults 50 and older, including an enhanced influenza vaccine in adults 65 and older.
"Combination vaccines have the potential to reduce the burden of respiratory viruses on health systems and pharmacies, as well as offer people more convenient vaccination options that could improve compliance and provide stronger protection from seasonal illnesses," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release on June 10, 2024.
"Moderna is the only company with a positive Phase 3 flu and COVID combination vaccine."
Moderna plans to present the Phase 3 clinical data for mRNA-1083 at an upcoming medical conference, submit them for publication, and engage regulators on the next steps.
mRNA-1083 comprises components of mRNA-1010, Moderna's vaccine candidate for seasonal influenza, and mRNA-1283, Moderna's next-generation COVID-19 vaccine candidate. Moderna says each investigational vaccine has independently demonstrated positive Phase 3 clinical trial results.
Our Trust Standards: Medical Advisory Committee