mRNA-Based Flu Shot Focuses on Improving Performance in Phase 2 Study
As influenza vaccine producers prepare for the 2024 - 2025 flu season, innovative vaccine candidates are progressing in clinical research, focused on enhancing efficacy and safety.
CureVac N.V. today announced interim data from an ongoing Phase 2 study, which is part of the combined Phase 1/2 study of its seasonal influenza vaccine candidate.
The purpose of this clinical trial (NCT05823974) is to find and confirm the dose and asses the reactogenicity, safety, and immune response of GlaxoSmithKline's (GSK) messenger RNA (mRNA)-based multivalent seasonal influenza vaccine (GSK4382276A) candidates administered in healthy younger and older adults.
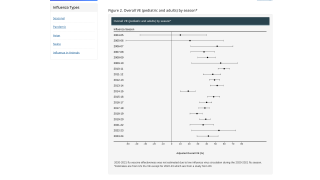
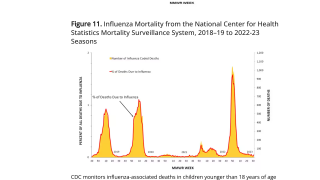
Results from the planned interim analysis showed that the multivalent vaccine candidate using CureVac's proprietary second-generation mRNA backbone boosted antibody titers against all encoded flu strains and across all age groups and tested dose levels, including the lowest tested dose.
"The Phase 2 interim data show that CureVac's highly effective and flexible mRNA technology platform puts us on the right track to advance our joint seasonal influenza vaccine program," said Dr. Myriam Mendila, Chief Development Officer of CureVac, in a press release on April 4, 2024.
"Results regarding influenza A strains were strong. Immunogenicity for B strains was also in line with our expectations in view of other initial mRNA-based clinical flu development programs."
"We are confident that planned optimizations will improve performance against these historically challenging influenza strains."
The multivalent candidate was selected from a comprehensive Phase 1 part, which tested vaccine candidates with up to eight separate mRNA constructs per candidate.
It was designed for broad antigen coverage, encoding antigens that matched all World Health Organization (WHO) recommended flu strains.
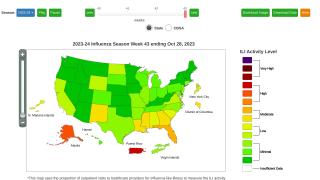
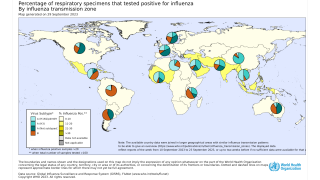
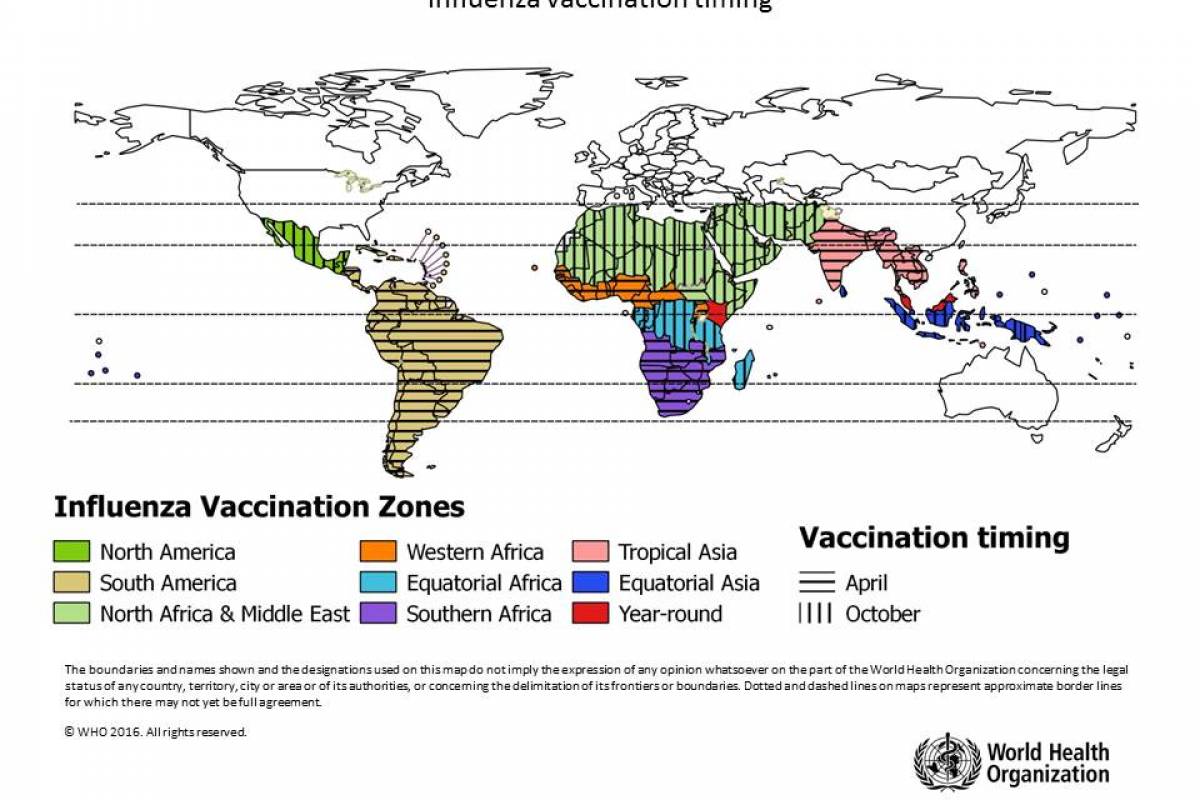
The WHO says flu shot campaigns should be timed according to local conditions.
Countries are encouraged to analyze local surveillance information to assess their seasonality pattern at both national and subnational levels, as appropriate, to make evidence-based decisions on the timing of vaccination campaigns.
Our Trust Standards: Medical Advisory Committee