Chikungunya Vaccine Candidate To Begin Testing in Europe

The European Medicines Agency (EMA) has agreed to pursue its proposed development plan for Emergent BioSolutions’ Chikungunya virus virus-like particle (CHIKV-VLP) vaccine candidate.
The company announced on January 13, 2020, it has proposed conducting a safety and immunogenicity Phase 3 clinical trial using Serum Neutralizing Antibodies (SNA) as an immune correlate of protection to predict clinical benefit of the CHIKV-VLP vaccine in Europe.
This is good news since there is no approved vaccine to prevent or medicine to treat Chikungunya virus infections.
Chikungunya outbreaks have occurred in countries in North and South America, Africa, Asia, Europe, and the Indian and Pacific Oceans and can be found in over 70 countries.
Furthermore, the US Centers for Disease Control and Prevention (CDC) says there is a risk that the virus will be imported to new areas by infected travelers.
As of January 9, 2020, a total of 134 chikungunya virus disease cases with illness onset in 2019 have been reported to ArboNET from 26 U.S. states.
Abbey Jenkins, senior vice president, and vaccines business unit head at Emergent said in a related press release, “As a leading provider of travel health vaccines, Emergent seeks to address the threat posed by this highly debilitating virus by defining a realistic and optimal path to bring to market a much-needed chikungunya vaccine that could potentially serve patients worldwide.”
“We look forward to continuing to work with regulators, including the U.S. Food and Drug Administration (FDA) with whom we had our End-of-Phase 2 meeting last December, as we plan to initiate a pivotal Phase 3 trial this year and define the approach for a post-approval confirmatory efficacy trial.”
The company’s proposed approach of using SNA as a surrogate endpoint is based on evidence from several preclinical, clinical and epidemiological studies.
This approach, presented by the company at a recent FDA Vaccines and Related Biological Products Advisory Committee meeting, is based on the premise that the feasibility of a field efficacy trial is complicated by the variable and unpredictable epidemiology of chikungunya disease.
Emergent’s CHIKV VLP vaccine candidate is currently being investigated in a Phase 2 parallel-group, randomized, double-blind, dose-finding study of approximately 430 healthy adults at three U.S. sites.
During 2019, the company presented updated results indicating that after a single dose, up to 98 percent of study participants produced a neutralizing antibody response against the chikungunya virus within seven days after vaccination.
Further, the immune response persisted for at least one year for subjects who received a single dose. The vaccine candidate was well-tolerated across all study arms and no significant vaccine-related safety concerns have been identified in analyses to date. Solicited adverse event profiles were similar across groups and mostly mild or moderate.
The CHIKV-VLP vaccine candidate is licensed from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. It received FDA Fast Track designation in May 2018 and EMA PRIME designation in September 2019.
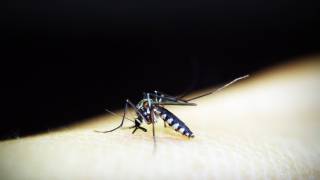
Chikungunya virus is spread to people through infected mosquitoes. Symptoms lasting 3-4 weeks include high-grade fever, joint pain, headache, muscle pain, joint swelling, or rash, while chronic arthritis occurs in a minority of patients.
Virus-like particle vaccines are multi-protein structures that mimic the organization and conformation of naturally occurring viruses without the viral genome.
Previous studies have shown that in general, other VLP vaccines are highly immunogenic, safe, and typically elicit high titer neutralizing antibodies, which are needed to protect against chikungunya virus.
Additional Chikungunya vaccine candidate information can be found on this page.
For more information visit Emergent BioSolutions.
Chikungunya vaccine news published by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee