Yellow Fever Vaccine Alert Issued by the UK

The Medicines & Healthcare products Regulatory Agency of the United Kingdom issued a notice saying ‘Due to an increased risk of life-threatening reactions, the yellow fever vaccine must not be given to anyone with a medical history of thymus dysfunction or who is immunosuppressed.’
This UK notice was issued on April 16, 2019, because ‘In recent months, we have been notified of 2 fatal adverse reactions to yellow fever vaccine.’
‘In one case, the yellow fever vaccine was given to a person with a history of thymectomy following a thymoma. In a 2nd case, the vaccine was given to a 67-year-old with no other known risk factors.’
‘Both patients died shortly after vaccination due to suspected yellow fever vaccine-associated viscerotropic disease (YEL-AVD).
The global reporting rate is around 1 case in every 1 million people vaccinated, with thymus disease, immunosuppression, and an age of 60 years and older, increases the risk.
Another serious risk of vaccination is vaccine-associated neurotropic disease (YEL-AND), which can occur at a similar rate and with the same risk factors. YEL-AND can present with a variety of neurological manifestations.
Previously, the UK issued an April 2016 Drug Safety Update and a reminder in November 2017 Drug Safety Update.
These Updates say ‘that live attenuated vaccines should not be given to people who are clinically immunosuppressed, either due to therapy, underlying illness, or pregnancy. This is because live vaccine strains can replicate and cause an extensive, severe, and sometimes fatal infection.’
For full prescribing information and warnings, precautions, and risks regarding the Stamaril vaccine, please refer to the Summary of Product Characteristics.
Any healthcare professional prescribing or administering the vaccine must ensure they are fully familiar with the up-to-date Summary of Product Characteristics.
More information and guidance on the yellow fever vaccine can also be found in the Green Book and the National Travel Health Network and Centre (NaTHNaC) website.
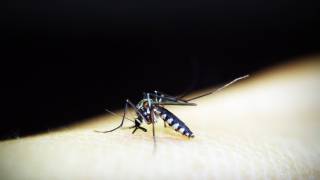
Protection from the yellow fever virus is important since about 15 percent of people who get yellow fever develop serious illness that can sometimes be fatal, says the US Centers for Disease Control and Prevention (CDC).
Yellow fever is a disease caused by a virus that is spread through mosquito bites. To prevent getting sick from the yellow fever virus, the CDC says to use insect repellent, wear long-sleeved shirts and long pants, and get vaccinated.
As of July 2018, the CDC published updated maps indicating which countries require yellow fever vaccination.
The CDC says its the clinician’s decision whether or not to vaccinate any traveler must take into account the traveler’s risk of being infected with YFV, country entry requirements, and individual risk factors for serious adverse events after yellow fever vaccination.
International travelers can find convenient locations to schedule a yellow fever vaccination appointment at least 10 days prior to departure by visiting Vax-Before-Travel.
As of July 2016, a completed International Certificate of Vaccination or Prophylaxis is valid for the lifetime of the vaccinee. Moreover, countries cannot require proof of revaccination against yellow fever as a condition of entry, even if the last vaccination was more than 10 years prior, says the CDC.
Relevant Links: CDC vaccination schedules, CDC vaccine price list, international travel alerts, and report vaccine side effects
Our Trust Standards: Medical Advisory Committee