Eradicate Malaria With Transformative Clinical Tools
To address malaria outbreaks in Africa, two approved malaria vaccines have been deployed in eight countries, with measurable success.
As 2024 progresses, additional African countries will launch malaria vaccination programs.
Additionally, passive immunization products, which have been tested for years, are now conducting late-stage clinical trials.
These experimental monoclonal antibody therapies offer significant value in protecting children, pregnant women, and immunocompromised individuals from contracting malaria.
According to a U.S. NIH press release on April 26, 2024, one injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children during the six-month malaria season, according to the results of a mid-stage clinical trial.
'We in the global malaria community are at a critical juncture in our journey toward eradication. Decades of experience deploying our existing interventions have made it clear that there is no single "silver bullet," wrote Trevor Mundel, M.D., Ph.D.
In an editorial published by the NEJM on April 26, 2024, Dr. Mundel continued,' Driving down morbidity and mortality requires that we use combinations of interventions across a range of product classes and that these combinations be uniquely tailored to local epidemiologic, demographic, environmental, and socioeconomic contexts.
Although our existing tools remain effective for now, the threat of drug and insecticide resistance necessitates a robust research-and-development pipeline capable of delivering improved versions of existing product classes and transformative tools that represent entirely new paradigms for treating and preventing malaria.
Furthermore, the intervention-delivery challenges inherent in accessing at-risk populations mean that these new tools — and the systems supporting them—must be amenable to widespread use and scale in some of the world's most challenging, hard-to-reach corners.
Innovation in monoclonal antibodies, long-acting injectable small-molecule drugs, and next-generation vaccines would give the world such potential tools.
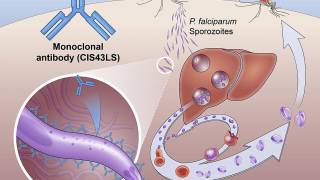
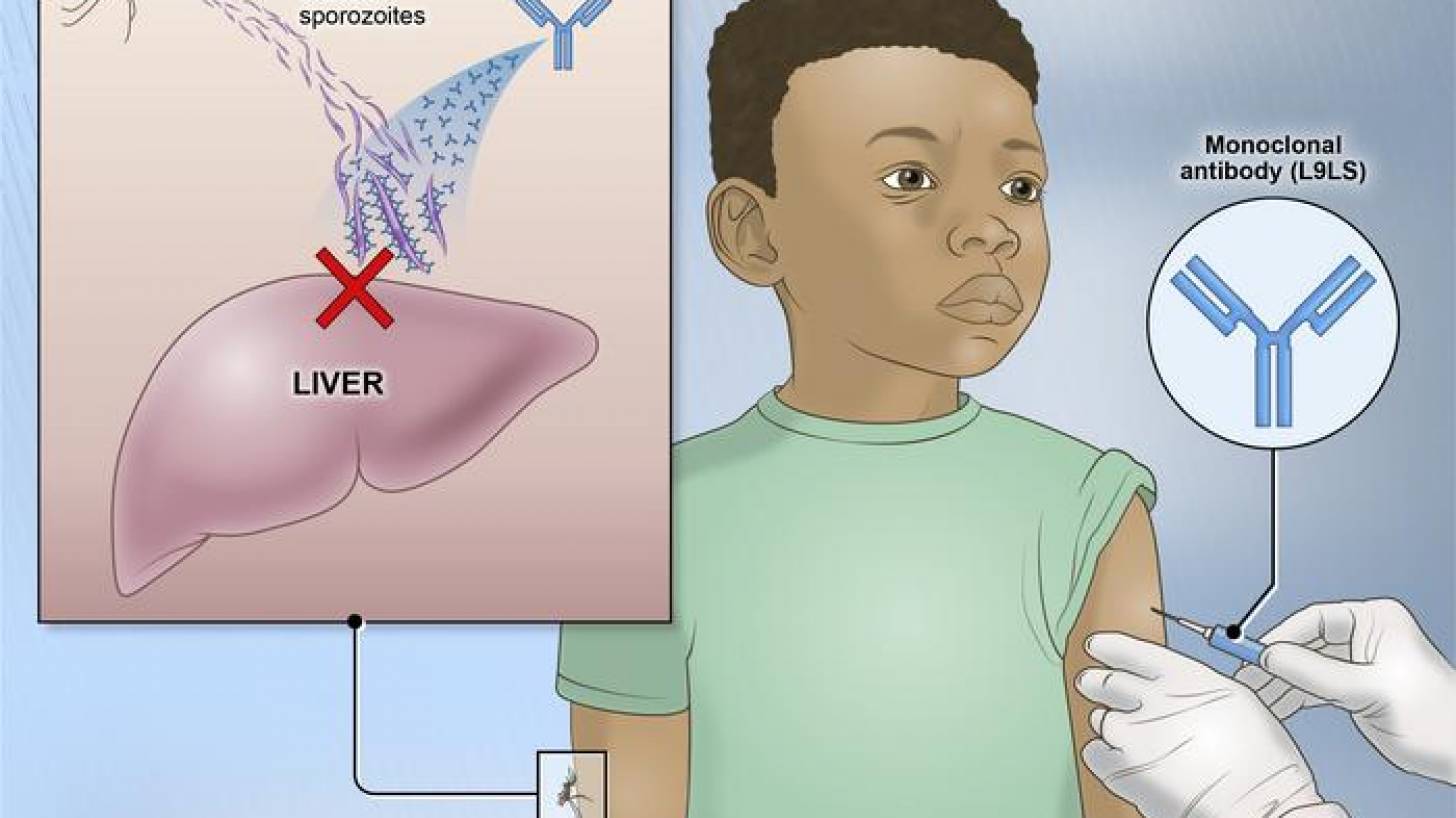
Monoclonal antibodies, the newest product class for the prevention of malaria and one that the Bill and Melinda Gates Foundation invests in, are molecules identified in and cloned from persons exposed to malaria using natural infection or vaccination.
The three monoclonal antibodies in clinical development target the highly conserved repeat region in the circumsporozoite protein expressed during the parasite's preerythrocytic stage.
These monoclonal antibodies block plasmodium sporozoites from establishing early infection in the liver, effectively preventing blood-stage infection, clinical disease, and onward transmission.
A monoclonal antibody or long-acting injectable drug that can deliver safe and cost-effective preventive efficacy from a single dose with a long duration of protection, as outlined in the WHO Preferred Product Characteristics.
These new product classes not only represent potentially highly effective, scalable, easy to implement, and durable tools for the prevention of malaria but also could conceivably be more feasible and effective prophylactic measures than either seasonal malaria chemoprevention or the currently recommended malaria vaccines — both in seasonal and perennial malaria-transmission settings, depending on the duration of protection.
The full, unedited editorial is posted at the NEJM link.
The use of antimalarial monoclonal antibodies needs to be considered in the context of deploying the WHO-approved RTS,S/AS01 and R21/Matrix-M vaccines. As of April 26, 2024, malaria vaccines are not offered in the United States.
Our Trust Standards: Medical Advisory Committee