New Ebola Vaccines Aspire to Achieve Older Version Success
As the current Sudan Ebolavirus outbreak in the Republic of Uganda comes to a close, researchers are scrambling to conduct clinical trials to determine if a new set of vaccine candidates can match the efficacy found in previous Ebola vaccines.
An Original Article recently published in the peer-reviewed New England Journal of Medicine (NEJM) confirmed that two existing Ebola vaccines trigger durable immune responses, with antibodies detectable a year after the first dose administration.
Published by the NEJM on December 16, 2022, this Article also confirmed the safety of three different regimens of Merck's Ervebo® and Johnson & Johnson's Zabdeno and Mvabea vaccines.
"The data collected during this clinical trial are valuable because they help confirm the safety and potential efficacy of the available vaccines, making it possible to refine the vaccination recommendations during both the Zaire ebolavirus epidemic and inter-epidemic periods in populations at risk," said the trial's principal investigator, Prof Yazdan Yazdanpanah of Paris Diderot University in France, in an article published by GAVI.
However, these vaccines are not focused on combating Uganda's current Sudan ebolavirus (SUDV) outbreak.
As of December 20, 2022, no approved vaccines protect people against SUDV.
However, the World Health Organization (WHO) stated on November 16, 2022, three candidate vaccines are under consideration.
Previously, the WHO announced that three candidate vaccines against SUDV arrived in Uganda and will be evaluated in human clinical trials called Tokomeza Ebola.
If approved, the SUDV vaccine candidates could be deployed should there be a sixth outbreak.
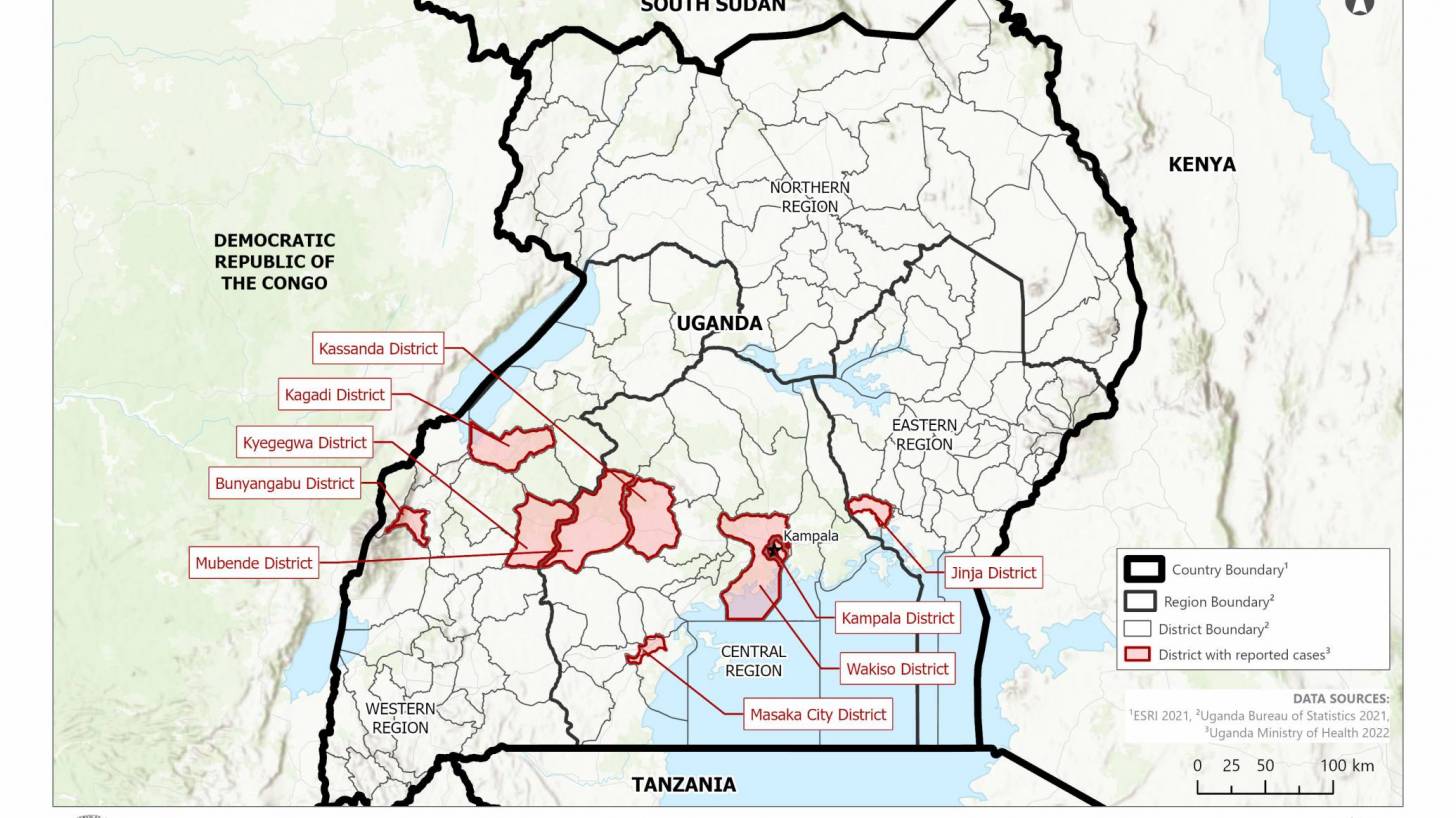
The current SUDV outbreak, which started on September 20, 2022, is the fifth outbreak in Uganda since 2000.
During the U.S. CDC's Clinician Outreach and Communication Activity (COCA) call on December 20, 2022, the presentation revealed 35 ill returning travelers from Uganda had been tested in the U.S. between September 20 – December 14, 2022.
Sudan virus testing was performed for 3/35 returning travelers at five airports, with all testing negative for the virus.
Currently, the CDC says the risk of Ebola importation into the U.S. is assessed as low for two reasons.
- There are few travelers and no direct flights to the U.S. from Uganda.
- Exit screening of air passengers is being conducted in Uganda.
The COCA call presenters did suggest including Ebola disease in the differential diagnosis for ill travelers who recently arrived from Uganda.
And be aware that malaria is the most common cause of undifferentiated fever after traveling to sub-Saharan Africa.
Going into the new year, the WHO recently announced that on January 12, 2023, it is organizing an expert consultation to discuss advances in evaluating the SUDV vaccine candidates.
Other Ebola outbreak news is posted at Vax-Before-Travel.com/Ebola.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee
























