West Nile Virus is Endemic in Phoenix

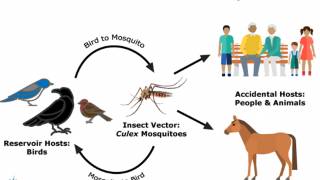
Since the West Nile virus (WNV) was first detected during 2003 in Maricopa County, Arizona, it has become endemic.
According to recent U.S. Centers for Disease Control and Prevention (CDC) data, the recent fifth WNV outbreak was the largest and generated the most significant number of related fatalities.
In 2021, Maricopa County, which includes the city of Phoenix, reported 1,487 WNV cases, 1,014 hospitalizations, and 101 (7%) deaths.
This unfortunate data compared with only 3 WNV cases in 2020.
Since its initial detection in the United States in 1999, WNV has become the leading cause of domestic arthropod-borne viral (arboviral) disease, says the CDC.
Spread by infected culex-species mosquitoes, WNV has caused more than 55,000 reported cases of human disease, more than 27,000 of them neuroinvasive, and 2,600 deaths between 1999 and 2021.
In the U.S., in addition to Arizona, the states of California and Colorado have been significantly impacted.
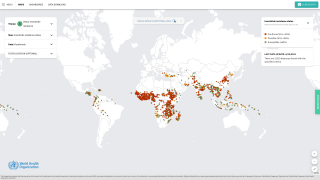
WNV is also an ongoing public health threat worldwide, with the largest recorded outbreak in Europe occurring in 2018.
Researchers in a Perspective published today by the New England Journal of Medicine stated, 'It is time to revisit the need for WNV vaccines for people.'
However, the U.S. FDA has not approved a WNV vaccine as of May 4, 2023.
Excerpts from this article are inserted below:
'Despite the development and expansion of WNV-specific mosquito surveillance and control programs over the past two decades, a consistently high disease burden is reported yearly in the U.S.
At a sub-national level, the occurrence of WNV is both geographically focal and sporadic, making it challenging to predict outbreaks and leading to regional and temporal variation in disease burden.
In addition to morbidity and mortality, WNV outbreaks create patient and social costs.
Between 2004 and 2017, a total of 3,109 California residents were hospitalized with WNV, with estimated hospital costs averaging $59.9 million per year.
Primary prevention of WNV includes personal protective measures to reduce vector exposure and community-based mosquito control programs. But these approaches have limitations and generally substandard results.
Proactive strategies, such as larvicide application and intensive, early-season adult mosquito control, are preferable but challenging to implement because of complexities in establishing evidence-based vector indexes, action thresholds, and variability in jurisdictional vector surveillance and resources.
Although several veterinary vaccines have been licensed, WNV vaccines for humans have not progressed beyond phase 2 clinical trials.
Human clinical studies have been conducted with several vaccine candidates.
Several factors have hindered WNV vaccines from progressing into later stages of clinical development, including challenges with designing and implementing efficacy studies, potential vaccine safety concerns, and anticipated costs of WNV vaccine programs.
Traditionally, large-scale phase 3, randomized clinical trials are needed to show efficacy; however, the sporadic and unpredictable nature of WNV disease outbreaks makes it challenging to select geographic areas and prepare for vaccine efficacy trials before WNV activity is detected during a given season.
In addition, severe disease is observed primarily in a subset of the population (older persons or those with certain underlying medical conditions).
Infection prevention would be challenging to assess because viremia is short-lived, and measurement of the WNV-specific antibodies required to confirm infection would need to differentiate between vaccine- and infection-induced immunity.
Concerns have also been raised over vaccine safety, particularly with live attenuated virus vaccine candidates.
Although early clinical trials with WNV chimeric vaccines did not find that older participants had vaccine-associated severe adverse events, the number of participants vaccinated was limited.
Another potential safety concern is antibody-dependent enhancement, which is observed with other flaviviruses and related vaccines.
The challenges to WNV vaccine development are not unique and could be addressed with approaches similar to those used for other vaccines.
To address the issue of cost barriers, an age- and incidence-based vaccine program could substantially improve cost-effectiveness while still leading to disease reduction.
Such a strategy was used successfully to implement the hepatitis A vaccine.
Our experience over the past two decades has demonstrated that current prevention strategies are not enough to reduce the ongoing WNV disease burden.
WNV vaccination would be more effective in preventing WNV disease and related deaths, wrote these researchers.
Note: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC.
The unedited NEJM article is posted at this link.
Our Trust Standards: Medical Advisory Committee