Technically, the HPAI A(H5N1) Outbreak Risk Remains Low

Although avian influenza (bird flu) viruses usually do not infect people, there have been some rare cases of human infection.
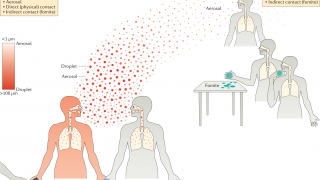
Human infections with bird flu viruses can happen when the virus gets into a person's eyes, nose, or mouth or is inhaled.
To clarify recent reports, the U.S. Centers for Disease Control and Prevention (CDC) today published a technical summary of an analysis conducted on the genomic sequences of viruses linked to an outbreak of highly pathogenic avian influenza (HPAI) A(H5N1) viruses in Texas.
HPAI A(H5N1) clade 2.3.4.4b viruses have been circulating globally in wild birds in the United States since late 2021. These HAPI viruses have caused commercial and backyard poultry outbreaks, with spillover resulting in sporadic infections in mammals.
As of April 2, 2024, the analysis results confirm that the risk to the general public associated with the ongoing HPAI A(H5N1) outbreak remains low.
The CDC stated while minor changes were identified in the virus sequence from the patient specimen compared to the viral sequences from cattle, both cattle and human sequences maintain primarily avian genetic characteristics and, for the most part, lack changes that would make them better adapted to infect mammals.
Avian influenza viruses can undergo changes in a host as they replicate after infection.
The genome for the human isolate from Texas had one change (PB2 E627K) that is known to be associated with viral adaptation to mammalian hosts and which has been detected before in people and other mammals infected with HPAI A(H5N1) virus and other avian influenza subtypes (e.g., H7N9).
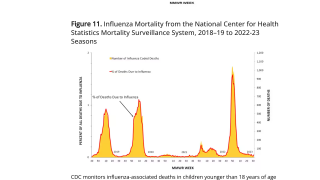
But there is no evidence of bird flu viruses spreading among people.
In addition to dairy cows, several mammals, such as Bears, cats, dogs, and seals, have recently been infected with this type of bird flu.
Furthermore, there are no markers known to be associated with influenza antiviral resistance found in the virus sequences from the patient's specimen, and the virus is very closely related to two existing HPAI A(H5N1) candidate vaccine viruses that are already available to manufacturers, and which could be used to make vaccine if needed.
The U.S. Food and Drug Administration authorized the Audenz™ (Influenza A(H5N1) Monovalent Vaccine, Adjuvanted) cell-based vaccine on January 31, 2020.
Overall, the genetic analysis of HPAI A(H5N1) viruses in Texas supports the CDC's conclusion that the human health risk currently remains low.
More details are available in this CDC technical summary, linked here.
On April 28, 2022, the state of Colorado reported an influenza A (H5) virus infection in a man. The CDC confirmed that this 'Montrose County, Colorado patient is the first human detection of any influenza A(H5) virus in the U.S. since 2016.
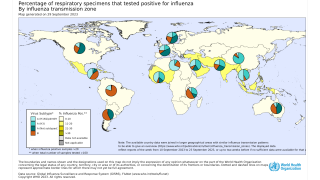
The World Health Organization (WHO) says available epidemiological and virological evidence currently suggests that influenza A(H5) viruses have not acquired the ability to sustain transmission among humans.
The WHO posted the cumulative worldwide number of confirmed human cases of avian influenza A(H5N1) reported (2003-2022).
Our Trust Standards: Medical Advisory Committee