Zika Test Approved for Blood Donations
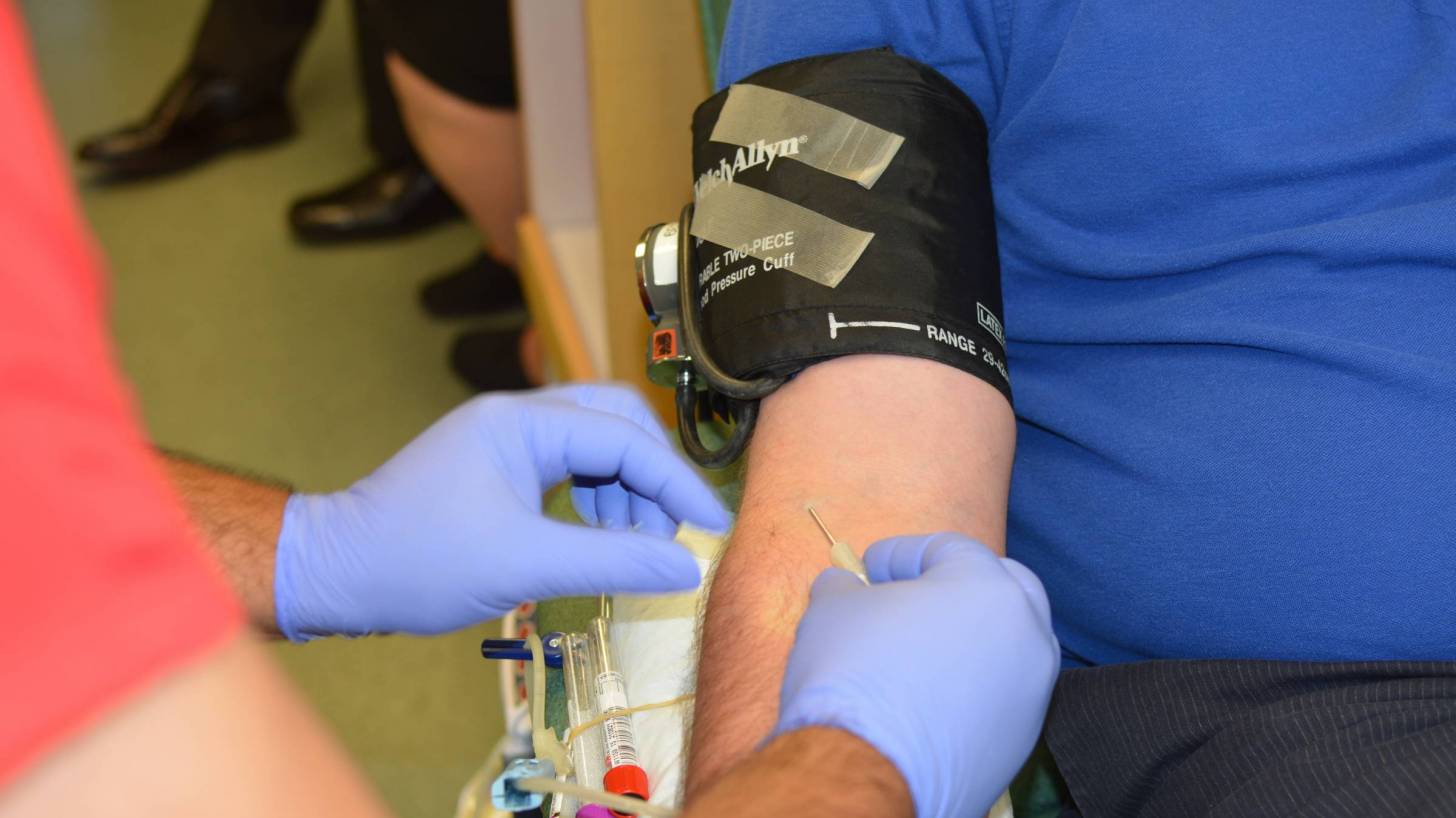
The cobas Zika test, a qualitative nucleic acid test for the detection of Zika virus RNA was approved by the U.S. Food and Drug Administration (FDA).
The cobas Zika test is intended for use by blood collection establishments to detect Zika virus in blood donations, not for the individual diagnosis of Zika virus infection.
"Today’s action by the FDA represents the first approval of a Zika virus detection test for use with screening the nation’s blood supply,"said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
"Screening blood donations for the Zika virus is critical to preventing infected donations from entering the U.S. blood supply,” said Dr. Marks.
In August 2016, the FDA issued a final guidance document recommending that all states and territories screen individual units of whole blood and blood components with an investigational blood screening test available under an investigational new drug (IND) application, or a licensed (approved) test when available.
Before today, several blood collection establishments used the cobas Zika test under IND in order to follow the recommendations in the FDA’s 2016 guidance document.
The test’s clinical specificity was evaluated by testing individual samples from blood donations at five external laboratory sites, resulting in clinical specificity of more than 99 percent.
The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. The cobas Zika test, cobas 6800, and cobas 8800 systems are manufactured by Roche Molecular Systems, Inc.
During July 2017, the Centers for Disease Control and Prevention (CDC) updated its recommendations for pregnant women with Zika symptoms and pregnant women without symptoms.
The change focuses on one of the most frequently used Zika tests which detects immunoglobulin M (IgM) antibodies and is more likely to yield a false positive test result.
Recent studies reported that Zika IgM antibodies might be detected for months after infection, limiting the ability to tell if the infection occurred before or during pregnancy.
Our Trust Standards: Medical Advisory Committee