mRNA Bird Flu Vaccine Candidate Launches Study in the U.S.

CureVac N.V. today announced the start of the Phase 1 part of a combined Phase 1/2 clinical trial of an investigational influenza A (H5N1) pre-pandemic vaccine candidate developed in collaboration with GSK.
In the initial Phase 1 dose-escalation part of the study, up to five dose levels will be assessed compared to a placebo control. The study will be conducted in the United States.
The monovalent vaccine candidate is based on CureVac's proprietary second-generation messenger ribonucleic acid (mRNA) backbone and encodes an influenza A H5-antigen.
Dr. Myriam Mendila, CureVac's Chief Development Officer, commented in a press release on April 24, 2024, "This clinical milestone, in collaboration with GSK, expands the application of our mRNA technology into an additional indication in infectious diseases and addresses the need to be prepared for potential future pandemics."
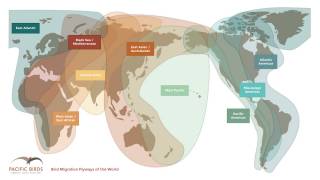
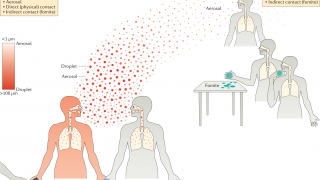
The H5N1 avian influenza virus is considered a potential pandemic threat. It is known to sporadically cross-species from its original bird host to other animal hosts, such as bears, cows, foxes, and humans worldwide.
The combined Phase 1/2 study will evaluate the safety, reactogenicity, and immunogenicity of an investigational influenza A (H5N1) pre-pandemic vaccine candidate in healthy younger and older adults.
The broad CureVac-GSK infectious disease collaboration was first announced in July 2020.
As of April 2024, the U.S. government has approved a bird flu vaccine (Audenz) and invested hundreds of millions in preparing avian influenza vaccine candidates.
Our Trust Standards: Medical Advisory Committee