Novel Therapeutic Cancer Vaccine Induced T-cell Responses Against HPV16 Antigens

Transgene today announced that new data confirm the ability of this novel investigational therapeutic cancer vaccine to induce immune responses against human papillomavirus (HPV) 16 antigens that are associated with antitumor response.
TG4001 is an investigational viral vector-based therapeutic cancer vaccine.
It is being evaluated in a randomized controlled Phase II clinical study comparing TG4001 with avelumab to avelumab alone in patients with HPV16-positive anogenital tumors.
The data presented on June 5, 2023, were generated from 46 patients in both trial arms.
- TG4001 induced the priming of adaptive immunity
- 58% of patients receiving TG4001 + avelumab showed an increase of immune responses against HPV antigens versus 9% in the avelumab arm. At baseline, immune responses against HPV antigens were limited to 4/46 patients.
- An immune response was detected at day 43 and tended to increase intensity at day 85.
- These data demonstrate that Transgene's TG4001 could induce a specific immune response against the antigens vectorized within this vaccine.
- 11 of the 13 patients with an immune response had either stable disease, partial or complete tumor response according to RECIST criteria.
- Remarkably, two case studies are presented, with patients exhibiting a strong E6 and E7 immune response while showing a complete clinical response.
- Transgene anticipates that the last patient will be randomized in the current Phase II clinical study in the first half of 2024. The final results will be communicated in 2024.
Dr. Alessandro Riva, MD, Chairman and CEO of Transgene, commented in a press release, "These data further confirm that our therapeutic vaccine TG4001 can induce clinically meaningful immune responses that are associated with antitumor response."
The abstract and poster can be accessed on the ASCO and Transgene websites.
TG4001 is an investigational therapeutic vaccine based on a non-propagative, highly attenuated Vaccinia vector, which is engineered to express HPV16 antigens (E6 & E7) and an adjuvant (IL-2).
TG4001 was designed to have a two-pronged antiviral approach: to alert the immune system specifically to cells presenting the HPV16 E6 and E7 antigens that can be found in HPV16-related tumors and to further stimulate the infection-clearing activity of the immune system through interleukin 2 (IL-2).
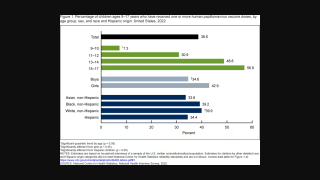
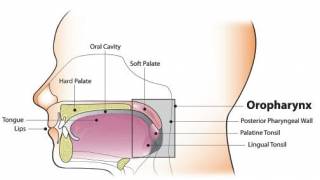
HPV is a group of more than 200 related viruses, some of which are spread through sex. Researchers previously confirmed that infection with HPV16 precedes the development of some head and neck cancers.
Other HPV cancer vaccine news is posted by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee