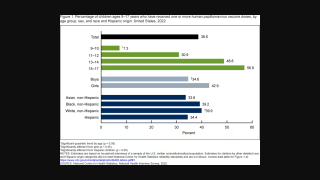
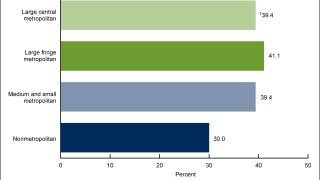
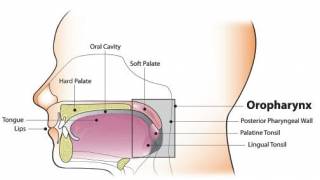
HPV-Positive Anogenital Cancer Vaccine Candidate Posts Positive Phase 2 Results

Transgene announced that following an interim analysis of its randomized controlled Phase II clinical study comparing TG4001 in combination with avelumab to avelumab alone in patients with HPV16- positive anogenital tumors, the Independent Data Monitoring Committee (IDMC) has recommended the study continue.
Transgene anticipates the last patient to be randomized in the trial in H1 2024.
TG4001 is designed to alert the immune system specifically to cells presenting the HPV16 E6 and E7 antigens found in HPV16-related tumors and to induce a specific cellular immune response against these cancer cells.
"We are very pleased with the outcome of this interim analysis," said Hedi Ben Brahim, CEO of Transgene, in a press release on November 2, 2022.
"The IDMC's recommendation to continue the study reinforces our confidence in TG4001, which follows promising data from our earlier Phase Ib/II trial."
To date, the treatment has been well tolerated. Adverse events are consistent with previous observations made in the Phase Ib/II trial.
TG4001 is based on an MVA vector engineered to express HPV16 antigens (E6 & E7) and interleukin 2 (IL-2).
Our Trust Standards: Medical Advisory Committee