Second-Gen Dengue Vaccine Remains Unavailable in the U.S.
Takeda, a Japanese company, announced its financial results today. The Company's press release confirmed that its second-generation dengue vaccine QDENGA® (TAK-003) remains unavailable in the United States.
In July 2023, Takeda announced that it voluntarily withdrew the Biologics License Application for TAK-003, following discussions with the U.S. Food and Drug Administration on aspects of data collection that could not be addressed within the current review cycle.
As of May 9, 2024, the plans for TAK-003 in the U.S. are unknown.
Therefore, the first-generation Dengvaxia® vaccine will continue to be the only option in Puerto Rico and southeast Florida, where dengue-infected mosquitoes are prevalent, and for international travelers visiting at-risk countries.
Additionally, Takeda disclosed that the World Health Organization (WHO) recently published a position paper (May 3, 2024) on dengue vaccines to include final guidance on using QDENGA in public vaccination programs.
As of 2024, QDENGA has been launched in 21 countries and is available in 17 European countries.
For example, Brazil has ordered over 5 million doses of QDENGA for immediate delivery and intends to produce its own dengue vaccine in late 2024.
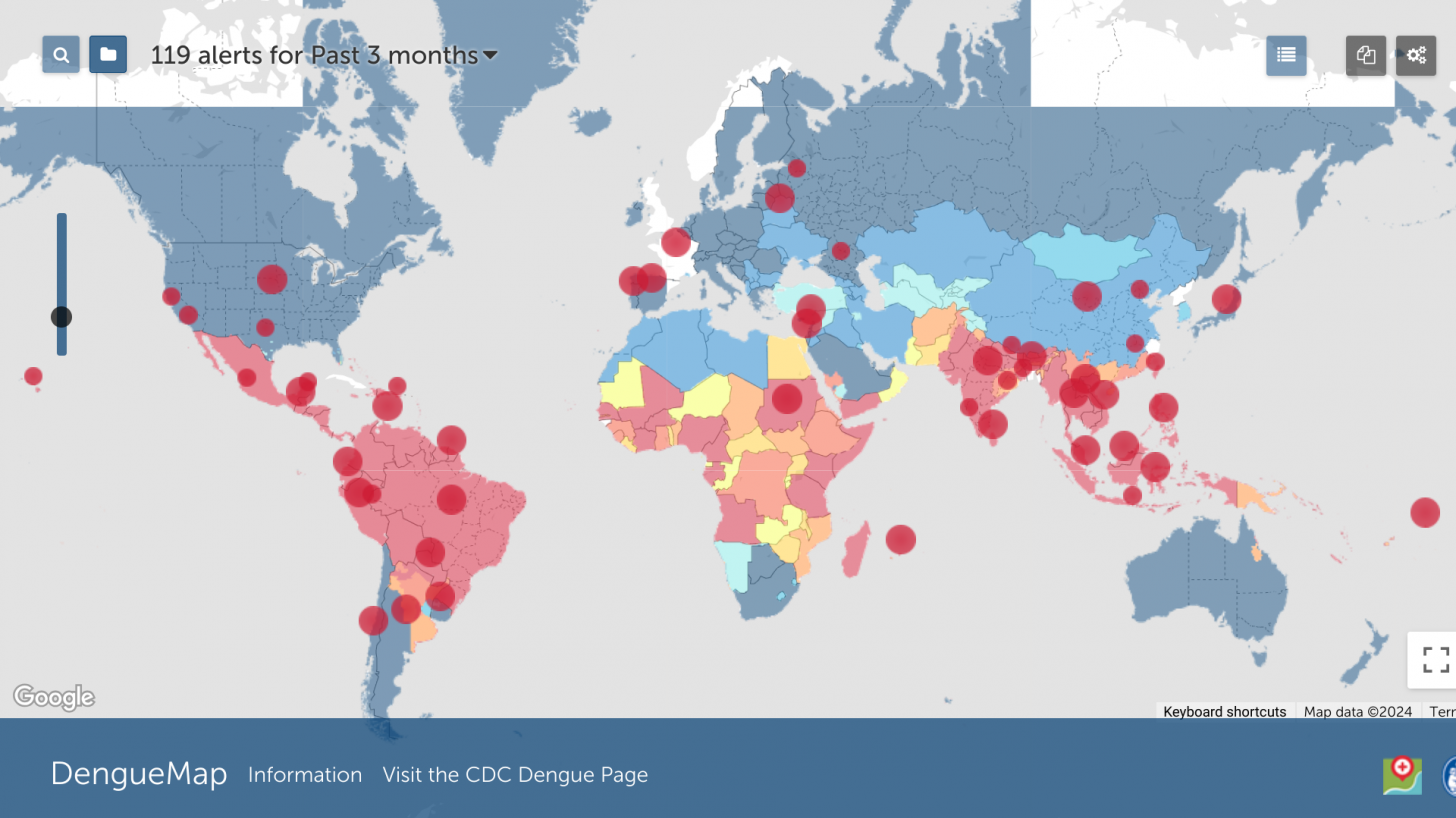
According to the WHO, dengue has quickly become endemic in about 125 countries. During 2023, over 5 million cases and more than 5,000 related deaths were reported during dengue outbreaks.
To alert international travelers of their potential dengue risk, the U.S. CDC updated and issued Travel Health Advisories in 2024.
Our Trust Standards: Medical Advisory Committee