FDA-Approved Yellow Fever Vaccine Returning to the USA

Sanofi Pasteur announced they expect the YF-VAX® (Yellow Fever Vaccine) to become available in the USA by the end of 2018.
Sanofi’s new state-of-the-art production facility has completed testing and the progress of the current validation activities for the YF-Vax vaccine has been confirmed, said Sanofi in a press statement.
Since 2017, Sanofi has worked with the FDA to distribute STAMARIL® Yellow Fever Vaccine through an Expanded Access Investigational New Drug Program (EAP) during the YF-VAX vaccine shortage.
The STAMARIL vaccine, manufactured by Sanofi Pasteur in France, is a live, attenuated yellow fever vaccine that is investigational/unlicensed in the USA, but it is registered and currently distributed in over 70 countries.
The EAP allows the importation and use of the STAMARIL vaccine in place of YF-VAX vaccine to fulfill USA yellow fever immunization demand.
An EAP is similar to a clinical trial, and a limited number of clinical sites can participate in this program. More than 250 locations have enrolled in the program, thus allowing them to immunize patients with yellow fever vaccine.
"It is critical to protect yourself ten days or more before traveling to yellow fever endemic areas," said Chris Felton, PharmD, at Brookshires Grocery pharmacy.
"Even though Stamaril is in the FDA’s “investigational new drug program”, it is not a new vaccine. Stamaril has been used in many countries for decades. Like YF-Vax, Stamaril is a live vaccine, and both vaccines have similar efficacy and safety profiles."
"If the YF-Vax vaccine is not available at your local pharmacy or clinic, a pharmacist can help you find one of the locations authorized to administer Stamaril, " said Felton.
Providers and patients may also visit CDC Travelers' Health for information about which countries require yellow fever vaccination for entry and for which countries the CDC recommends yellow fever vaccination.
Additionally, the Pan American Health Organization, Regional Office of WHO for the Americas (PAHO-WHO) reiterated its previous recommendation that international travelers be vaccinated at least 10 days prior to traveling to or visiting areas where yellow fever is circulating.
Confirmed cases of yellow fever in unvaccinated travelers underscore the need for countries to ensure wide dissemination of these recommendations.
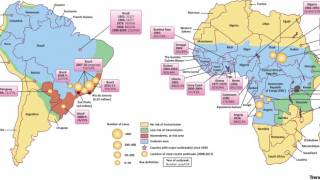
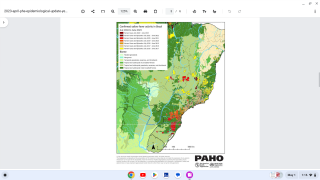
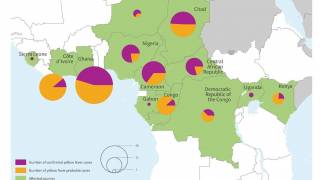
Between January 2016 to January 2018, seven countries and territories in the Region of the Americas have reported cases of yellow fever: Bolivia, Brazil, Colombia, Ecuador, French Guiana, Peru, and Suriname.
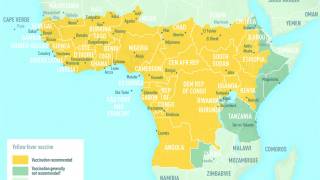
The WHO recommends vaccination for international travelers to 21 Brazilian states, considered to be at risk for yellow fever transmission, including the states of São Paulo and Rio de Janeiro.
The determination of new areas considered to be at risk for yellow fever transmission is an ongoing process, which WHO closely monitors and provides regular updates. For the latest information on yellow fever updates, visit this link.
YF-VAX Vaccine Prescribing Information
STAMARIL Prescribing Information
Our Trust Standards: Medical Advisory Committee