Leprosy Vaccine Moves Into Human Trials

The first leprosy vaccine candidate, developed specifically for prevention, is beginning a Phase 1 clinical trial.
This vaccine candidate, called LepVax, was developed through screening and identifying Mycobacterium leprae proteins that trigger an effective immune response.
Study participants can’t contract leprosy from the LepVax vaccine because it doesn’t contain the Mycobacterium leprae.
This clinical trial is launching in Madison, Wisconsin, where 24 people ages 18 to 55 are being recruited to participate. In this study, healthy people will be given three injections of the vaccine over two months, with some receiving a higher dose than others.
Leprosy is caused by the bacteria Mycobacterium leprae, a cousin of the bacteria that causes tuberculosis.
Characterized by the World Health Organization (WHO) as a "neglected tropical disease," leprosy (Hansen's disease) is one of the most ancient diseases known to humankind.
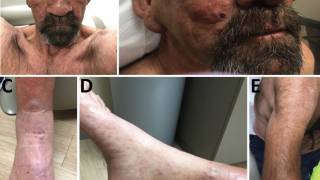
According to the WHO, nearly 250,000 people worldwide have leprosy, which causes disfiguration of the skin and mucous membranes as well as progressive and incurable nerve damage.
During 2016, a total of 2,743 cases of leprosy relapse were reported by 54 countries. Brazil reported 1,431 cases, India 536 and Indonesia 229, with the remaining 547 cases were reported by 51 countries.
While drug therapy exists for leprosy, it must be taken for many months, has many side effects and often is given too late to reverse the permanent damage caused by the bacterial infection.
Since 1995 WHO has provided free medications to all leprosy patients in the world.
"We are thrilled that after 15 years and an investment of over $5.1 million, a leprosy-specific vaccine is beginning a Phase I clinical trial," says Bill Simmons, President and CEO of American Leprosy Missions.
Scientists produced a fusion of four leprosy proteins, which is combined with IDRI's proprietary immune-stimulating adjuvant. With recent developments in vaccine technology, IDRI scientists can now induce protective responses even after infection, meaning it could be possible to prevent disease development in people already infected with the leprosy bacterium.
"The development has been complicated by the fact that the organism that causes leprosy cannot be grown in culture in the laboratory," Reed explained. "This is a unique example of a vaccine produced by totally synthetic methods."
Early results from the Phase I study should be available in 2018.
IDRI had developed technology to enable a rapid, affordable, point-of-care diagnostic test, which was funded by NIAID (grant numbers 1R43AI066613-01A1 and 2R44AI066613-02).
In addition to IDRI and American Leprosy Missions, a variety of partners are taking part in efforts to eliminate leprosy, including Novartis and the Novartis Foundation.
As a nonprofit global health organization, IDRI (Infectious Disease Research Institute) takes a comprehensive approach to combat infectious diseases, combining the high-quality science of a research organization with the product development capabilities of a biotech company to create new diagnostics, drugs and vaccines.
Our Trust Standards: Medical Advisory Committee
- IDIR
- Leprosy elimination
- Microbiology of M.leprae
- Factsheet
- Leprosy
- Promising new leprosy vaccine moves into human trials
- Leprosy: improved reporting, active case-finding and enhanced data collection reveal hidden cases
- Immune-stimulating complexes as adjuvants for inducing local and systemic immunity after oral immunization with protein antigens
- Covance site of first human study of vaccine for leprosy