Lyme Disease Vaccine Candidate Advances in Phase 2 Studies

A French biotech company announced that it has completed patient recruitment of the Phase 2 studies for its Lyme disease vaccine candidate, VLA15.
Valneva SE said in a press release published on September 30, 2019, comprising immunogenicity and safety data from these studies are expected in mid-2020, will support the dose and vaccination schedule to be used in Phase 3 clinical studies.
This is good news since VLA15 is currently the only active vaccine program in clinical development against Lyme disease. The program was granted Fast Track designation by the U.S. Food and Drug Administration (FDA) in July 2017.
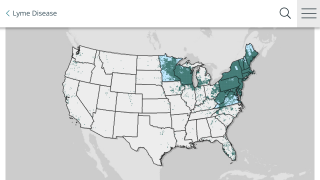
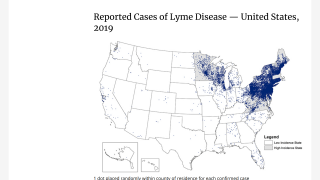
The medical need for a Lyme disease vaccine is steadily increasing as the disease footprint continues to widens in the USA and in Europe.
And it is also a costly medical burden. The U.S. health care system spends between $712 million and $1.3 billion a year and $3,000 per patient, related to Lyme disease.
In the USA, ‘the geographic distribution of high incidence areas with Lyme disease appears to be expanding into additional states, based on data reported to the National Notifiable Disease Surveillance System(NNDSS).
Wolfgang Bender, M.D., Ph.D., Chief Medical Officer of Valneva, commented, “We are extremely pleased that all Phase 2 subjects have been recruited as planned and on target. We are making great progress in developing our Lyme disease vaccine candidate with the aim of addressing this significant unmet medical need.”
Recent Lyme Disease vaccine news
- Lyme Disease Carrying Ticks Are Outside This Summer
- LymePrEP Could Stop Lyme Disease Before It Starts
- Tick-Borne Disease SFTS Vaccine Candidate Found ‘Protective’
A total of 819 healthy adults 18 to 65 years of age have been recruited for Phase 2 development in two ongoing studies, which are as follows:
- VLA15-201 is a randomized, observer-blind, placebo-controlled trial conducted at trial sites in the U.S. and Europe. VLA15 is tested as an alum adjuvanted formulation and administered intramuscularly in three injections, given one month apart at Days 1, 29 and 57. Subjects will be followed for one year, with the main immunogenicity readout one month after the third immunization on Day 85 (primary endpoint).
- VLA15-202 is the 2nd of two planned Phase 2 studies. It is a randomized, observer-blind, placebo-controlled trial conducted at trial sites in the US. 246 subjects will receive one of two dosage levels of VLA15 or placebo. Subjects will be followed for 18 months, with the main immunogenicity readout on Day 208 (primary endpoint).
Lyme disease is a systemic infection caused by Borrelia bacteria transmitted to humans by infected Ixodes ticks. It is considered the most common vector-borne illness in the Northern Hemisphere.
>>Search for a Clinical Trial<<
According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 300,000 Americans are diagnosed with Lyme disease each year with at least a further 200,000 cases in Europe.
Early symptoms of Lyme disease are often overlooked or misinterpreted. Left untreated, the disease can disseminate and cause more serious complications affecting the joints, the heart or the nervous system.
Previously, the CDC issued new recommendations for a 2-tier test for Lyme disease diagnosis.
On August 15, 2019, the CDC said ‘when cleared by the FDA, serologic assays that utilize enzyme immunoassay rather than western immunoblot assay in a 2-test format are acceptable alternatives for the laboratory diagnosis of Lyme disease.’
Additionally, ‘clinicians and laboratories should consider serologic tests cleared by the FDA as CDC-recommended procedures for Lyme disease serodiagnosis.’
Furthermore, on July 29, 2019, ‘the FDA cleared several Lyme disease serologic assays with new indications for use based on a modified 2-test methodology.
>> Fast Lyme Disease Testing <<
The FDA says the CDC recommendations should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate.
The CDC says healthcare providers should consider:
- The signs and symptoms of Lyme disease
- The likelihood that the patient has been exposed to infected black-legged ticks
- The possibility that other illnesses may cause similar symptoms
- Results of laboratory tests, when indicated.
Valneva is a biotech company based in Saint-Herblain, France, developing and commercializing vaccines for infectious diseases with major unmet needs. Valneva’s portfolio includes two commercial vaccines for travelers: IXIARO®/JESPECT® indicated for the prevention of Japanese encephalitis and DUKORAL® indicated for the prevention of cholera and, in some countries, prevention of diarrhea caused by ETEC.
Lyme Disease Vaccine information published by Precision Vaccinations
Our Trust Standards: Medical Advisory Committee