CRISPR-Cas9 Gene-Editing May Lead to a Zika Treatment

A new study published by Tel Aviv University (TAU) has identified genes that protect cells from the Zika virus.
This study’s findings may lead to the development of a treatment for the Zika virus, which is not available today.
This research published online May 29, 2019, was based on a modification of the CRISPR-Cas9 gene-editing technique.
CRISPR-Cas9 is a naturally occurring bacterial genome editing system that has been adapted to gene editing in mammalian cells. The system is based on the bacterial enzyme Cas9, which can locate and modify specific locations along the human genome.
A modification of this system, known as CRISPR activation, is accomplished by genetically changing Cas9 in a way that enables the expression of specific genes in their original DNA locations.
“CRISPR activation can be used to identify genes protecting against viral infection,” said Dr. Ella H. Sklan of TAU’s Sackler School of Medicine. “We used this adapted system to activate every gene in the genome in cultured cells. We then infected the cells with the Zika virus."
While most cells die following the infection, some survived due to the over-expression of some protective genes. These researchers used next-generation sequencing and bioinformatic analysis to identify a number of genes that enabled survival, focusing on one of these genes called IFI6.
“IFI6 showed high levels of protection against the Zika virus both by protecting cells from infection and by preventing cell death,” Dr. Sklan said in a press release on July 25, 2019.
A previous screen conducted by another research group had identified this gene with respect to its role vis-à-vis other viruses.
“If its yet unknown mode of action can be mimicked, it may one day serve as the basis for the development of a novel antiviral therapy to fight the Zika virus or related infections.”
Together with Dr. Nabila Jabrane-Ferrat of The French National Center for Scientific Research, Dr. Sklan moved the study of the identified genes into Zika-infected human placenta tissues, which serve as a gateway for viral transmission to the fetus.
These genes were induced following infection, indicating they might play a protective role in this tissue as well.
“Our results provide a better understanding of key host factors that protect cells from ZIKV infection and might assist in identifying novel antiviral targets,” concludes Dr. Sklan.
Moving forward, the researchers hope to discover the mechanism by which the IFI6 gene inhibits infection.
Zika vaccine news
- Cuba Visitors Returned With the Zika Virus
- 49 Zika Cases Returned to America in 2019
- Zika Remains a Worldwide Health Risk to Pregnant Women
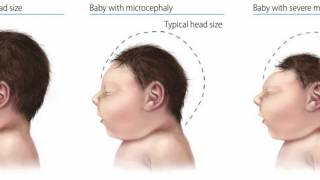
- Zika Exposure While Pregnant Associated with Microcephaly
- US Government Makes 2nd Zika Investment
- New Legislation May Protect the USA From Zika
- Where Are Californians Acquiring the Zika Virus?
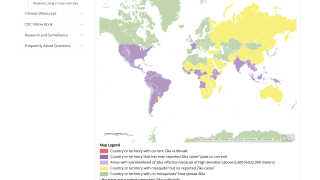
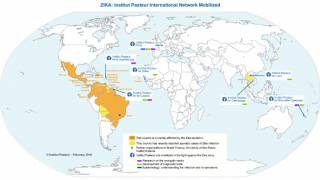
The Zika virus has affected over 60 million people, mostly in South America. It has potentially devastating consequences for pregnant women and their unborn children, many of whom are born with severe microcephaly and other developmental and neurological abnormalities.
During 2019, USA residents have contracted the Zika virus while traveling abroad, while Puerto Rico has reported locally-acquired cases.
The research for the study was conducted by Dr. Anna Dukhovny of TAU’s Sackler School of Medicine, and bioinformatics analysis conducted by Kevin Lamkiewicz of Friedrich Schiller University. Part of the study was conducted during Dr. Sklan’s sabbatical in Prof. Jae Jung’s lab at the University of Southern California.
Our Trust Standards: Medical Advisory Committee