Yellow Fever Vaccine Availability Increasing During 2019

Sanofi Pasteur, the producer of the YF-VAX® (Yellow Fever Vaccine) now expects supply to return in the USA by mid-2019.
This supply change is based upon the construction and validation of a new Yellow Fever vaccine manufacturing facility.
Sanofi said in a press statement that “With the most recent testing complete and the progress of the current validation activities confirmed, we adjusted our expectations for the return to the supply of YF-VAX vaccine.”
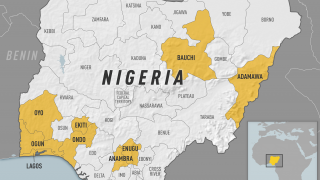
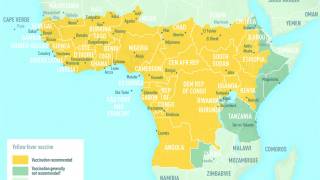
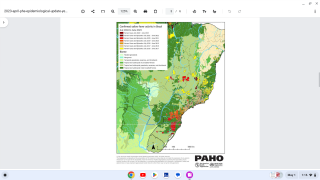
Sanofi’s goal is to support continued access to yellow fever vaccination for travelers to international destinations where yellow fever vaccine is required or recommended, such as Brazil.
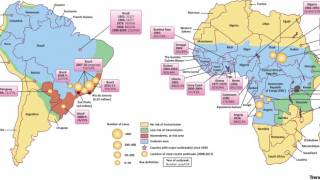
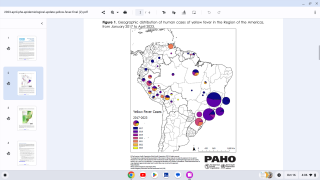
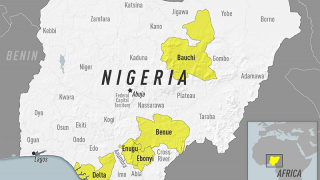
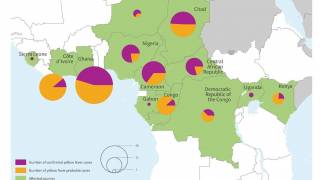
According to the U.S. Centers for Disease Control and Prevention (CDC), about 15 percent of people who get yellow fever develop serious illness that can be fatal. Yellow fever virus is a mosquito-borne flavivirus that causes yellow fever, an acute infectious disease.
Sanofi has worked with the FDA to distribute the STAMARIL® (Yellow Fever Vaccine [Live]) through an Expanded Access Program (EAP) until production of the YF-VAX vaccine resumes, in its new facility.
The EAP allows the importation and use of STAMARIL® (Yellow Fever Vaccine [Live]) in place of YF-VAX vaccine to fulfill U.S. yellow fever immunization demand until production of YF-VAX vaccine resumes.
An EAP is similar to a clinical trial, and a limited number of clinical sites can participate in this program. More than 250 locations have been selected to include sites that immunize the most patients with YF-VAX vaccine.
Pharmacists, healthcare providers, and patients can find locations that can administer STAMARIL vaccine by visiting the CDC web page.
STAMARIL is registered and currently distributed in over 70 countries.
Providers and patients may also visit CDC Travel for information about which countries require yellow fever vaccination for entry and for which countries the CDC recommends yellow fever vaccination.
Currently, only 4 yellow fever vaccine manufacturers are World Health Organization prequalified:
- Brazil’s Bio-Manguinhos,
- Russian Federation’s Institute of Poliomyelitis and Viral Encephalitides,
- Senegal’s Institut Pasteur,
- France’s Sanofi Pasteur.
To schedule a travel vaccination appointment, please visit Vax-Before-Travel.
The CDC Vaccine Price List provides private sector vaccine prices for general information.
And, vaccine discounts can be found here.
Vaccines, like any medicine, can have side effects. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
Our Trust Standards: Medical Advisory Committee