1 Billion Africans to Receive Yellow Fever Vaccination

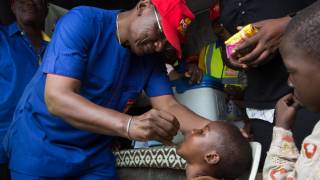
Nearly 1 billion people will be vaccinated against yellow fever in 27 high-risk African countries by 2026, said the World Health Organization (WHO).
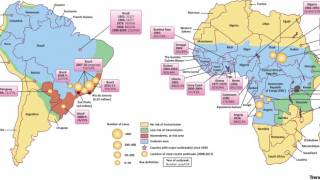
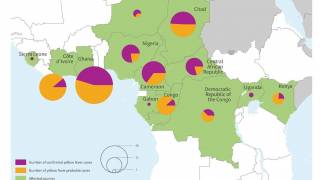
This WHO announcement comes after outbreaks of Yellow fever virus in densely populated cities in Angola and the Democratic Republic of Congo, which caused 400 deaths in 2016.
Because of increased international travel, the yellow fever virus could be on the verge of spreading to various countries. The number of confirmed yellow fever cases in unvaccinated international travelers has reached 10, reported the WHO.
“The yellow fever vaccine serves two major purposes. It will protect travelers from a potentially deadly disease, but it also protects susceptible and vulnerable regions from an outbreak,” said Chris Felton, PharmD MTM Clinical Pharmacist with Brookshire Grocery Company.
“Travelers need to check the yellow fever requirement for each country they will be visiting….even if it is only a short layover. Talk to a pharmacist well in advance of departure and get any necessary vaccines,” Felton explained.
***Schedule your appointment today for recommended vaccines***
As of 2016, yellow fever vaccine had been introduced in routine infant immunization programmes in 35 of the 42 countries and territories at risk for yellow fever in Africa and the Americas. In these 42 countries and territories, vaccination coverage is estimated at 45%, says the WHO.
UNICEF will make the Stamaril vaccines available and provide support in vaccinating children through routine immunization, as well as during outbreaks of the disease.
Stefan Peterson, UNICEF’s Chief of Health said, "Given that almost half of the people to be vaccinated are children under 15 years of age, this campaign is critical to saving children’s lives, and would go a long way toward stamping out this disease."
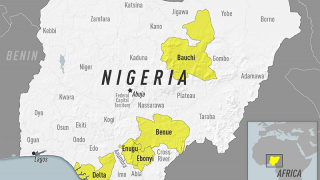
The commitment is part of the Eliminate Yellow fever Epidemics (EYE) in Africa strategy, which was launched by Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, Professor Isaac Folorunso Adewole, Nigeria’s Minister of Health and partners at a regional meeting in Abuja, Nigeria, on April 10, 2018.
Previous experience in West Africa demonstrates that the EYE strategy can work.
When Yellow fever re-emerged as a public health issue in the early 2000s, countries in the region controlled the epidemics through preventive mass campaigns combined with routine immunization. No yellow fever epidemics have been recorded in countries which successfully implemented this approach, says the WHO.
This implementation effort follows the endorsement of the strategy by African Ministers of Health at the 67th WHO regional committee in September 2017.
Dr. Tedros said, "With one injection we can protect a person for life against this dangerous pathogen. This unprecedented commitment by countries will ensure that by 2026 Africa is free of Yellow fever epidemics."
Yellow fever virus is a mosquito-borne flavivirus that causes yellow fever, an acute infectious disease that occurs in South America and sub-Saharan Africa. Most patients with yellow fever are asymptomatic.
But, among the 15 percent who develop severe illness, the yellow fever case fatality rate ranges up to 60 percent, says the WHO.
The objectives of the strategy include:
- protecting at-risk populations through preventive mass vaccination campaigns and routine immunization programmes,
- preventing international spread, and containing outbreaks rapidly,
- developing strong surveillance with robust laboratory networks is key to these efforts.
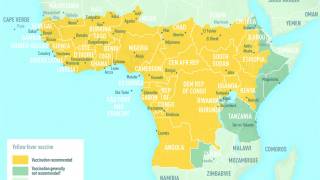
Yellow fever vaccine is recommended for people 9 months and older who are traveling to or living in areas at risk for yellow fever virus transmission in South America and Africa.
Sanofi Pasteur, the manufacturer of the only yellow fever vaccine (YF-Vax) licensed in the United States, announced on February 22, 2018, that YF-Vax for civilian use is expected to be available from the manufacturer again by the end of 2018.
Until then, Sanofi Pasteur received approval from the US Food and Drug Administration to make Stamaril available to USA residents.
Stamaril is distributed in more than 70 countries and is comparable in safety and efficacy to YF-Vax.
In the USA, various pharmacies offer the STAMARIL and YF-VAX vaccines, as well as other travel vaccines.
For most international travelers, a single dose of yellow fever vaccine provides long-lasting protection. However, some travelers may require a booster dose, says the WHO.
Reactions to yellow fever vaccine are generally mild and include headaches, muscle aches, and low-grade fevers. There have been reports of serious events following yellow fever vaccination.
You are encouraged to report any vaccine side effects to the FDA or CDC.
The CDC Vaccine Price List provides the private sector prices and general information, and discounts can be found here.
Our Trust Standards: Medical Advisory Committee
- Immunization coverage
- Sixty-seventh session of the WHO Regional Committee for Africa
- Announcement: Temporary Total Depletion of US Licensed Yellow Fever Vaccine Addressed
- YELLOW FEVER VACCINE INFORMATION
- Nearly one billion people in Africa to be protected against yellow fever by 2026
- Yellow Fever Vaccine
- Passports, Visas and Yellow Cards, All May Be Needed When Traveling