Yellow Fever Vaccine Shortage Announced

Health officials are warning that this country's supply of yellow fever vaccine will likely be completely depleted for the immunization of U.S. travelers by mid-2017.
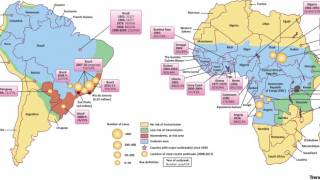
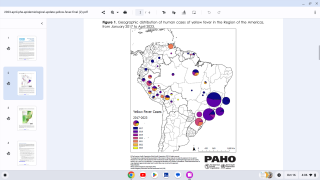
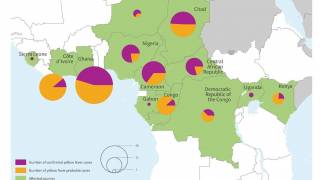
This vaccine shortage comes as multiple yellow fever outbreaks have occurred around the world, most recently reported in Brazil.
The Centers of Disease and Control and Prevention (CDC), the Food and Drug Administration (FDA), and Sanofi Pasteur are collaborating to ensure a continuous supply of yellow fever vaccine in the United States.
As part of this joint effort, Sanofi Pasteur submitted an expanded access investigational new drug (eIND) application to FDA in September 2016 to allow for the importation and use of an alternative yellow fever vaccine manufactured by Sanofi Pasteur France that has similar safety and efficacy compared to the U.S.-licensed vaccine.
That vaccine, Stamaril, is considered investigational and is not approved for use in the United States.
However, Stamaril is used in more than 70 other countries.
CDC and Sanofi Pasteur will continue to communicate with the public and other stakeholders, and the CDC will provide a list of locations that will be giving the replacement vaccine at a later date.
In the United States, only one yellow fever vaccine is licensed for use, YF-VAX (manufactured by Sanofi Pasteur). Approximately 500,000 doses are given every year to protect the Armed Forces and civilian travelers.
Domestic production of the Sanofi yellow fever vaccine in the United States should resume in 2018.
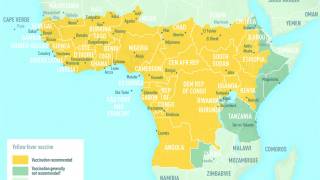
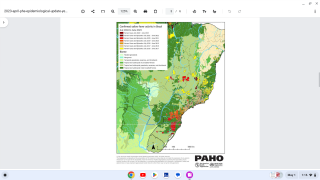
Yellow fever is an acute viral disease caused by infection with the yellow fever virus, a flavivirus primarily transmitted to humans through the bite of an infected mosquito. It is endemic to sub-Saharan Africa and tropical South America.
The case-fatality ratio is 20%–50% among the approximately 15% of infected persons who develop severe disease. In recent years, multiple yellow fever outbreaks in Angola, the Democratic Republic of the Congo, and, most recently, Brazil, have underscored the ongoing and substantial global burden of this disease.
Yellow fever can be prevented by a live-attenuated virus vaccine that produces protection in 80%–100% of patients within 10 days after vaccination.
For most travelers, only one lifetime dose is necessary.
The CDC recommends the vaccine for travelers visiting areas overseas with a yellow fever endemic or epidemic yellow fever. In addition, proof-of-vaccination is required for entry into certain countries as permitted by the International Health Regulations 2015.
To provide proof of vaccination, practitioners at yellow fever vaccination clinics must validate a traveler’s vaccine record using a proof-of-vaccination stamp. CDC has regulatory authority over the designation of U.S. yellow fever vaccination clinics
“The hardest thing for international travelers to do, is to find a certified provider to purchase the vaccine. The yellow fever vaccine requires special state approval to administer the product,” said Rannon Ching, Pharm.D, a travel vaccine specialist at Tarrytown Pharmacy in Austin, TX.
“Many of Tarrytown Pharmacy patients are requesting this vaccine to visit areas for humanitarian purposes, in addition to sightseeing. This vaccine helps protect people when traveling to at risk area,” said Ching.
As of April 2017, approximately 250 clinics were targeted for inclusion. This is a sizable reduction from the estimated 4,000 civilian clinics currently providing YF-VAX.
In 2015, approximately 8 million U.S. residents traveled to 42 countries with a yellow fever.
Our Trust Standards: Medical Advisory Committee