RSV Vaccine Candidates Approach the Starting Gate

After decades of false starts, new research indicates four Respiratory Syncytial Virus (RSV) vaccine candidates are nearing the completion of late-stage trials.
According to the U.S. CDC, RSV vaccines could drastically reduce hospital and intensive-care admissions for young children and seniors.
Unfortunately, an earlier version created sickness in children.
RSV infections are usually most severe in infants under two months old, who encounter the virus for the first time.
A series of clinical trials tested a vaccine made from inactivated RSV in children in the 1960s. Tragically, the vaccine candidate worsened the disease in vaccinated children when they were later naturally infected with RSV, reported the journal Nature in 2008.
These ‘breakthrough’ infections caused severe lung disease that hospitalized most of the children in the trial and killed two.
And for seniors, RSV infections are dangerous as immune systems weaken.
According to the CDC, RSV can sometimes lead to pneumonia (infection of the lungs) and congestive heart failure (when the heart can’t pump blood and oxygen to the body’s tissues).
Each year, it is estimated that more than 177,000 seniors are hospitalized, and 14,000 of them die in the U.S. due to RSV infection.
Because of the acute medical need for RSV vaccines, regulatory agencies in the United States and Europe are now prioritizing them for expedited review.
“It’s been eight years since the prefusion F protein conformation was elucidated, and now we’re all in phase III trials based on that fundamental discovery,” commented Christine Shaw, vice-president of early-development programs at Moderna Inc., in a Nature news article published in December 2021.
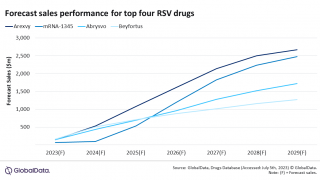
The good news is RSV vaccine candidates are approaching late-stage study completion.
Moderna recently announced its mRNA-1345 RSV vaccine candidate was granted U.S. FDA Fast Track designation for seniors. And it is conducting both phase 1 and 3 studies. This vaccine encodes for a prefusion F glycoprotein, eliciting a superior neutralizing antibody response.
And Pfizer Inc. recently announced that its RSV vaccine candidate RSVpreF received a second Breakthrough Therapy Designation (BTD) from the U.S. Food and Drug Administration (FDA) to prevent lower respiratory tract disease in children caused by RSV.
RSVpreF previously received a BTD from the FDA on March 2, 2022, for the prevention of RSV-associated lower respiratory tract illness in infants from birth up to six months of age by active immunization of pregnant women.
A third vaccine candidate conducting phase 3 study is GSK's RSVPreF3 OA, a single-dose RSV vaccine candidate that is currently conducting a phase 3 study in adults aged 60 and above. It contains a recombinant subunit pre-fusion RSV antigen combined with GSK's proprietary AS01 adjuvant.
A fourth vaccine is Bavarian Nordic's MVA-BN RSV vaccine candidate, which incorporates five different RSV antigens to stimulate a broad immune response against both RSV subtypes (A and B), thus mimicking the immune response observed following a natural reaction to an RSV infection.
Hopefully, these vaccines will soon become available, as new research is quantifying their need:
- The JAMA Network published an Original Investigation in Feb. 2022 that suggests RSV poses a greater risk to infants than influenza, while both are associated with substantial mortality among elderly individuals.
- The journal PNAS published research on March 14, 2022, which found that administering an RSV vaccine to pregnant mothers reduced antimicrobial prescribing among their infants by 12.9% over the first three months.
Furthermore, the National Foundation for Infectious Diseases issued a Call to Action on January 27, 2022, urging stronger public health focus and increased awareness of RSV in the U.S.
The report, Call to Action: Reducing the Burden of RSV across the Lifespan, addresses the underappreciated impact of RSV and outlines strategic priorities to drive progress in RSV surveillance, diagnosis, prevention, and treatment.
And once approved by the U.S. FDA, these innovative RSV vaccines could be available at pediatric clinics and clinical pharmacies throughout the U.S.
Other RSV vaccine news is published at this weblink.
PrecisionVaccinations published fact-checked, research-based vaccine news.
Note: This feature article aggregated content from various sources and was manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee