Zika Virus Blood Donation Testing Guidelines Revised by FDA
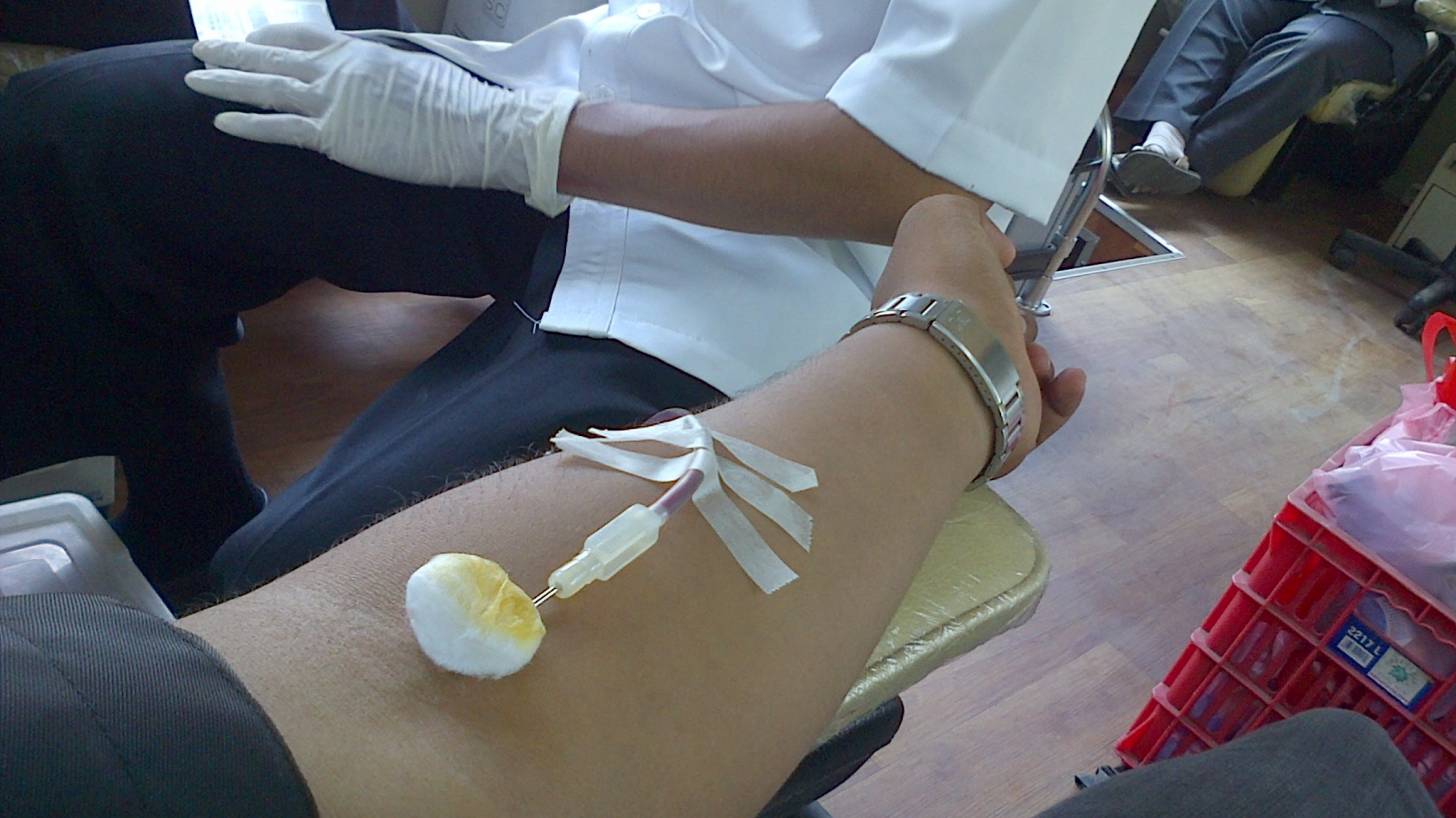
The Food and Drug Administration (FDA) announced a revised blood donation testing process for the Zika virus.
Given the significant decrease in the number of Zika virus infections in the USA and its territories, the FDA is moving away from testing each individual donation, to testing pooled blood donations.
Pooled blood testing is usually more cost-effective and less burdensome for blood collection establishments, said the FDA.
However, the FDA will continue to monitor the situation closely, and as appropriate, reconsider what measures are needed to maintain the safety of the blood supply.
Although a Zika virus infection is self-limiting, concerns about its impact on pregnant women and related birth defects are well established.
“To help protect the blood supply from infectious diseases, the FDA continually assesses the latest scientific information available to ensure that our blood deferral and testing recommendations best safeguard the millions of people who depend on blood donations every year,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, in a press release.
On July 29, 2016, the Centers for Disease Control and Prevention (CDC) that likely local mosquito-borne Zika virus transmission has been reported in the continental United States. Locally transmitted Zika virus has also been reported in the Commonwealth of Puerto Rico, the U.S. Virgin Islands, and American Samoa.
The revised guidance explains the basis for the FDA’s determination that pooled testing of donations using a screening test licensed for such use by the FDA is a sufficient method for complying with these regulations and effectively reducing the risk of Zika Virus transmission, unless there is an increased risk of local mosquito-borne transmission of Zika virus in a specific geographic area that would trigger individual donation testing in that location.
Alternatively, blood establishments may use an FDA-approved pathogen-reduction device for plasma and certain platelet products.
The change comes after careful consideration of all available scientific evidence, including consultation with other public health agencies, and following the recommendations of the December 2017 meeting of the Blood Products Advisory Committee.
The FDA said it is confident that today’s recommendations will continue to ensure the safety of the U.S. blood supply by reducing the risk of transmission of Zika virus while reducing the burden of testing for blood establishments.
Separately, on April 2, 2018, the Texas Department of State Health Services updated its statewide Zika testing guidance for asymptomatic pregnant women.
Zika virus is transmitted primarily by the Aedes mosquito, but it can also be spread by other routes, including by blood and sexual contact.
Zika virus can also be associated with Guillain-Barre syndrome and severe neurological complications.
Moreover, Zika virus infection during pregnancy can cause serious birth defects and is associated with other adverse pregnancy outcomes.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices.
Our Trust Standards: Medical Advisory Committee
- Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components
- FDA announces revised guidance on the testing of donated blood and blood components for Zika virus
- America’s Zika Family Tree Originated in Brazil
- Infectious Disease Carrying Mosquitoes Love These Cities
- Updated Zika Testing Guidelines April 2, 2018
- Zika Virus Response Updates from FDA
- Differentiating Between Dengue and Zika Just Became Easier