Norovirus Vaccines
Norovirus Vaccines 2024
While vaccines against norovirus diseases are in demand, developing a broadly effective vaccine in 2024 remains difficult, owing to the vast genetic and antigenic diversity of noroviruses, which have multiple co-circulated variants of different genotypes. As of March 27, 2024, the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the U.K. had not approved a norovirus vaccine. The World Health Organization (WHO) has not pre-qualified any norovirus vaccine in 2024.
Norovirus Vaccine Candidates
HIL-214 is a bivalent vaccine candidate in development for preventing moderate-to-severe acute gastroenteritis (AGE) caused by norovirus infection. HIL-214 consists of virus-like particles designed to mimic the structure of two significant genotypes of norovirus, GI.1 and GII.4, and are co-formulated with an alum adjuvant. Topline safety and clinical efficacy data from NEST-IN1 are expected in the first quarter of 2024.
HilleVax's HIL-216 hexavalent virus-like particle (VLP) vaccine candidate for norovirus includes VLPs for six of the most common norovirus genotypes, including GI.1, GII.2, GII.3, GII.4, GII.6, and GII.17. The U.S. FDA cleared an Investigational New Drug application for HIL-216 in September 2023.
Moderna Inc. is developing two multivalent virus-like particle vaccines, mRNA-1403 (trivalent) and mRNA-1405 (pentavalent). The randomized, observer-blind, placebo-controlled Phase 1 clinical trial of the mRNA-1403 vaccine is currently enrolling participants. An interim analysis showed that a single dose of mRNA-1403 elicited a robust immune response across all dose levels evaluated with a clinically acceptable reactogenicity and safety profile. The Company confirmed on March 27, 2024, it is advancing mRNA-1403 toward a pivotal Phase 3 trial.
Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd. is conducting a phase 1/2a clinical trial evaluating the safety and tolerability of the tetravalent recombinant Norovirus vaccine at different doses. This candidate uses the Pichia pastoris expression system, which establishes the virus-like particles (VLP) recombinant engineered bacteria for expressing norovirus epidemic strains, which are then cult.
Vaxart Inc.'s orally administered bivalent GI.1/GII.4 norovirus oral vaccine candidate is conducting a phase 2b study. It is differentiated from other norovirus vaccine candidates because it generates systemic and mucosal immunity delivered through the mouth and is stable at room temperature. On July 6, 2023, and September 6, 2023, Vaxart announced positive phase 2b clinical trial results. Vaxart announced on December 21, 2023, that it completed enrollment and dosing of a Phase 1 clinical trial (VXA-NVV-108) focused on lactating mothers.
Cocrystal Pharma, Inc. oral, first-in-class pan-norovirus and pan-coronavirus 3CL protease inhibitor CDI-988 dosed the first subjects in a Phase 1 clinical trial on September 29, 2023. Recent CDI-988 in vitro studies showed potent broad-spectrum antiviral activity against a panel of pandemic GII.4 norovirus proteases and a favorable pharmacokinetic property targeting the gastrointestinal tract.
Norovirus Outbreaks
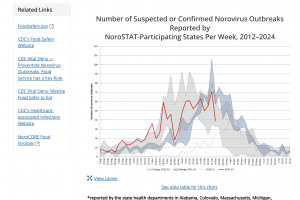
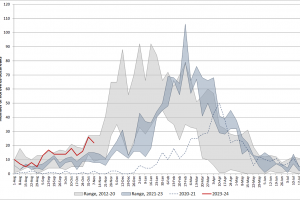
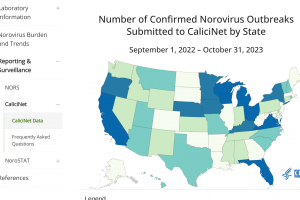
Based on CaliciNet Data, the U.S. Centers for Disease Control and Prevention CDC published the Norovirus Outbreak Map of confirmed outbreaks in 2024. The Wastewater SCAN Norovirus map is updated in 2024. The CDC also posts Norovirus National Trends. The CDC says the GII.4 proteases have caused most norovirus outbreaks since 20—however, non-GII.Four viruses, such as GII.17 and GII.2, have replaced GII.4 viruses in several Asian countries. Separately, the U.K. Health Security Agency publishes summaries of norovirus and rotavirus laboratory surveillance and enteric virus outbreaks in hospital and community settings in England during the 2022 to 2023 season.
Norovirus Outbreaks Cruise Ships
The U.S. CDC posts and the UK identified norovirus outbreaks and alerts for the cruise ship industry. On February 8, 2024, the CDC reported that the Cruise Ship Queen Victoria (V405) reported a substantial gastrointestinal illness that impacted the crew and passengers. There were 12 norovirus outbreaks on cruise ships in 2023.