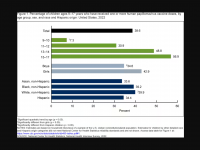
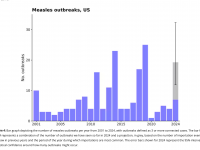
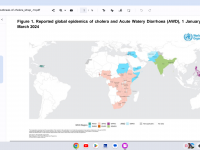
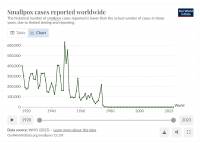
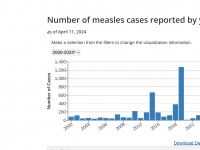
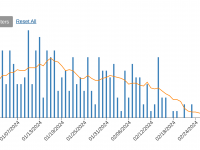
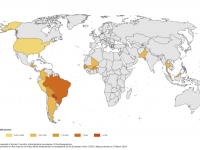
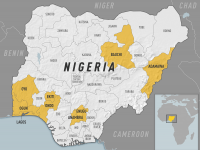
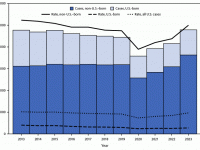
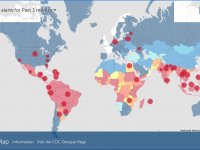
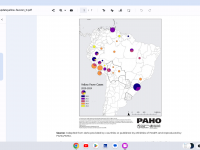
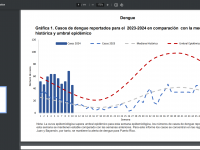
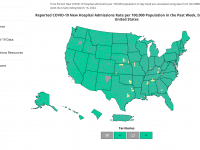
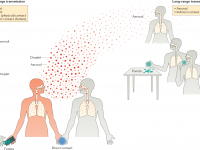
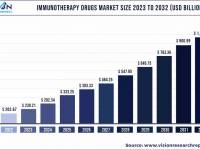
The Florida Department of Health published Arbovirus Surveillance Update #15, which discloses various mosquito-borne diseases reported this year. As of April 13, 2024, countries in southern Florida confirmed these mosquito-transmitted diseases: Chikungunya - Three cases of... READ →
Precision Vaccinations
/ April 23rd, 2024Eliminating fake vaccine news with pharmacist, nurse, and physician review.
For more articles, try our search.