New Polio Vaccines Display Good Immune Response Against Mutated Strain

Clinical studies indicate the new poliovirus vaccine may have the potential to overcome outbreaks caused by a mutated polio strain linked to the oral vaccine.
According to studies published by The Lancet on December 9, 2020, scientists have developed the first poliovirus vaccine against a mutated form of the disease that is causing disease outbreaks across Africa and Asia.
These scientists evaluated the two nOPV2 candidates in parallel clinical studies in adults, young children, and infants in two different countries to assess their safety and immunogenicity compared with the licensed Sabin mOPV2 they are designed to replace.
Designed to be more genetically stable than the licensed Sabin oral vaccine, the new vaccine appears to be as safe and provides similar immune responses when tested in healthy adults, children, and infants, according to the research published in The Lancet.
The new vaccine, known as nOPV2, is directed against poliovirus type 2, and has improved genetic stability, and is less likely to mutate and revert into a form of the virus that can cause infection and paralytic disease.
Based on the results from these phase 2 clinical trials, the nOPV2 vaccine has received an Emergency Use Listing (EUL) recommendation from WHO making it the first vaccine ever to go through this pathway that is meant for global health emergencies.
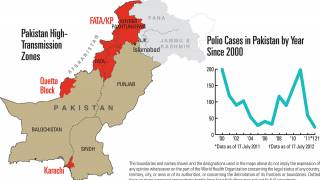
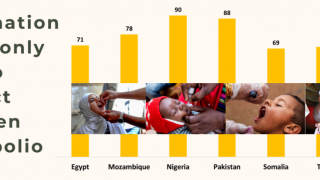
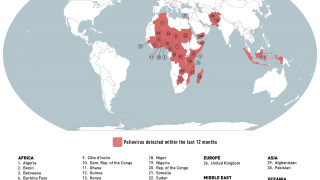
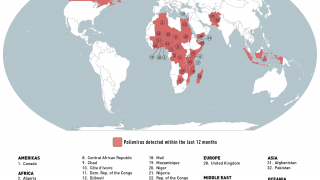
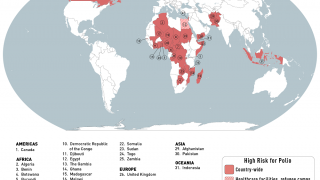
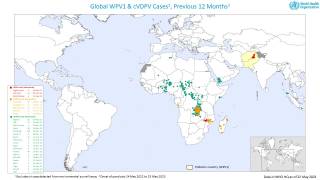
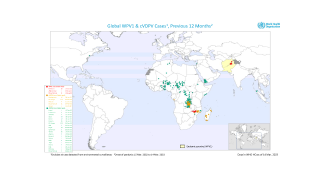
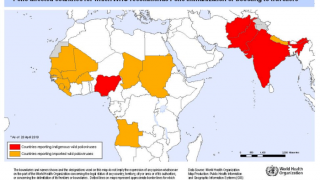
The aim is to now use the vaccine for outbreak response for vaccine-derived poliovirus that is increasing across Africa as well as Afghanistan, Pakistan, the Philippines, Malaysia, and other countries.
While there is no cure, polio can be prevented through vaccination against the three polio types, 1, 2, and 3 in trivalent vaccines.
Currently, outbreaks are being tackled using the original Sabin oral vaccine for type 2 polio, which risks seeding further outbreaks in areas of persistently low immunization coverage.
Lead author of the study in adults, Professor Pierre Van Damme from the University of Antwerp in Belgium says: "Millions of people potentially have no immunity to the spread of the vaccine-derived virus, which is caused when the weakened live virus in oral polio vaccines mutates and regains its ability to become infectious, cause disease, and spread in communities with low vaccination rates.”
“The nOPV2 vaccine appears at least as safe and effective as the Sabin vaccine and genetically more stable, and could be a key breakthrough towards a polio-free world."
In low- and middle-income countries, the oral vaccine is used because of its low cost and ease of use, needing only two drops per dose, and because it induces intestinal immunity which decreases transmission of the live virus in conditions of poor sanitation.
In most high-income countries, a more expensive, injectable vaccine, which contains inactivated viruses incapable of causing disease is used, but it does not induce intestinal immunity, which means that vaccinated individuals can still shed the orally-acquired virus.
In recent years, eradication efforts have been challenged by increasing outbreaks of circulating vaccine-derived poliovirus (cVDPV), with over 90% of cases caused by mutations in a strain of type 2 poliovirus.
This risk is what led to the global withdrawal of the type 2 component of the oral trivalent vaccine in May 2016. However, this effort failed to curb all type 2 poliovirus transmission, with numbers of type 2 cVDPV cases increasing from 71 in 2018 to 739 cases in 2020 (as of Dec 3, 2020).
In response, WHO maintained its categorization of type 2 cVDPV as a Public Health Emergency of International Concern on October 22, 2020.
Polio is a highly infectious viral disease, which mainly affects children under five, with around one in 200 infections resulting in paralysis. Of those paralyzed, up to 10 percent die when their breathing muscles become immobilized.
To address this global emergency, the Global Polio Eradication Initiative supported the development of more genetically stable type 2 polioviruses for vaccines.
Like the licensed Sabin type 2 monovalent oral polio vaccine (mOPV2), the new oral vaccine candidates (nOPV2-c1 and nOPV2-c2) are derived from the live, infectious virus, but have been modified to reduce the likelihood of reverting to a type of the virus that can cause infection and disease in rare circumstances.
Because the original Sabin mOPV2 was withdrawn in 2016, before the nOPV2 candidates were developed, it was not possible to compare the vaccines at the same time.
For this reason, Belgian researchers did two randomized trials in healthy Belgian adults (aged 18-50 years): a prospective phase 4 study of Sabin mOPV2 from January to March 2016 (before global withdrawal) to provide historical control data, and a phase 2 study of both nOPV2 candidates from October 2018 to February 2019. Participants in both trials had previously been fully vaccinated against polio as part of routine immunizations.
In the historical control study, 100 participants were enrolled and received one or two doses of Sabin mOPV2. In the phase 2 study, 250 volunteers were randomly assigned to receive either one or two doses of a nOPV2 candidate or a placebo, 28 days apart. Immune responses were assessed on the day of vaccination, then 4 weeks after their first and second vaccinations. Participants recorded any adverse events throughout the study.
Results showed that both nOPV2 candidates were as safe and well-tolerated as mOPV2, and generated a similar immune response--achieving 100% seroprotection (an antibody response capable of preventing infection) after one dose for both nOPV2 candidates compared with 97% and 98% for one or two doses of mOPV2.
Following these promising safety results in fully vaccinated adults, researchers conducted two randomized trials in Panama in healthy young children (aged 1-4 years) and infants (aged 18-22 weeks) who had been previously immunized with existing vaccines: a historical control phase 4 study with Sabin mOPV2 from October 2015 to April 2016 (before global withdrawal), and a phase 2 study with low and high doses of the two nOPV2 candidates between September 2018 and September 2019.
Overall, 150 children (50 in the control study and 100 in the nOPV2 study) and 684 infants (110 controls and 574 in the nOPV2 study) were enrolled and received at least one study vaccination. Immune responses were assessed 1 and 4 weeks after their first vaccination and 4 weeks after their second vaccination. Parents recorded any adverse events for 28 days after each vaccination.
The results indicate that in infants, one or two doses of both nOPV2 candidates were safe and well-tolerated, with similar immunogenicity to mOPV2--despite mOPV2 recipients having higher baseline immunity at the start of the study.
The nOPV2 candidates also had lower stool shedding rates 28 days after vaccination than seen with mOPV2 in the control study.
Lead author of the studies in children and infants, Dr. Ricardo Rüttimann from the US non-profit Fighting Infectious Diseases in Emerging Countries, says, "Our findings show that both novel OPV2 candidates are safe, well-tolerated, and immunogenic in young children and infants.”
“However, on-going monitoring in the field and additional studies will strengthen these observations to confirm the safety and effectiveness of the vaccine as it gets used under EUL for outbreak response.”
“Ultimately, in order to eradicate all forms of polio, countries must improve routine and supplementary immunization campaigns to ensure enough children are reached to boost population immunity high enough so that the virus can no longer survive."
The study authors note some limitations to their studies, including that they included just 1,200 individuals.
Additional information will still be needed on longer-term safety and duration of protection. They also point out that although the studies were designed to be aligned as far as possible in terms of study centers, population, design, and analyses, a direct comparison was not possible because of the global withdrawal of mOPV2 in 2016.
The study in adults was sponsored by the University of Antwerp with funding support from the Bill & Melinda Gates Foundation.
The study in children and infants was sponsored by Fighting Infectious Diseases in Emerging Countries with funding support from the Bill & Melinda Gates Foundation.
No conflicts of interest were disclosed by these researchers.
PrecisionVaccinations publishes research-based vaccine development news.
Our Trust Standards: Medical Advisory Committee
- Poliovirus vaccine options: another step forward
- Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovir
- Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovir