New Polio Vaccine Recommendation Issued

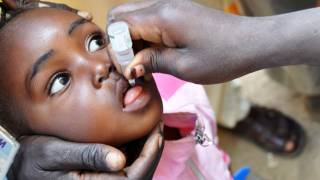
The World Health Organization’s (WHO) Prequalification program issued an Emergency Use Listing (EUL) recommendation for the type 2 novel oral polio vaccine (nOPV2).
On November 13, 2020, this announcement will enable the rollout of the nOPV2 vaccine for use in countries affected by circulating vaccine-derived poliovirus type 2 (cVDPV2) outbreaks.
The WHO stated: ‘clinical trials of the new vaccine have shown it to be safe and provide comparable protection against polio, as the currently used mOPV2 vaccine.
The vaccine has shown to be more genetically stable than mOPV2, making it significantly less likely to revert into a form that can cause paralysis in low immunity settings.
This means a reduced risk of seeding new cVDPV2 outbreaks compared to mOPV2, which remains a safe and effective vaccine that protects against polio and has successfully stopped cVDPV2 outbreaks in the past, says the WHO.
nOPV2 is a modified version of mOPV2 and has been in development for close to a decade, thanks to the collaboration of an extensive network of global experts.
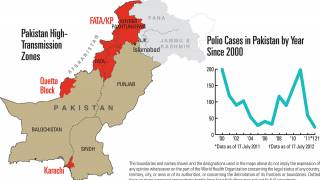
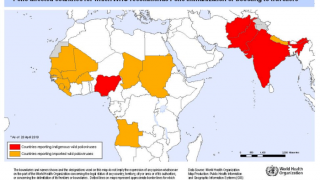
In light of ongoing cVDPV2 emergencies across countries in Africa and Asia, coupled with polio’s status as a Public Health Emergency of International Concern (PHEIC) since 2014, the WHO Executive Board urged the Member States in February 2020 to expedite the processes for authorizing the importation and use of nOPV2 under the EUL given data showing the vaccine’s promise against cVDPV2s.
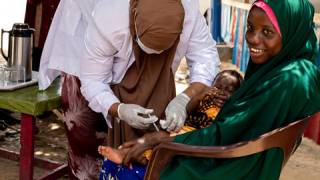
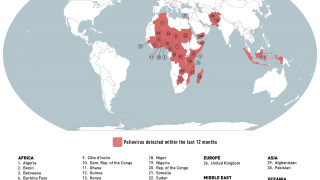
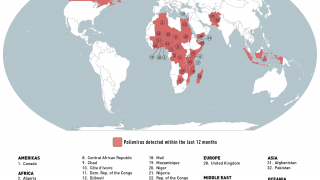
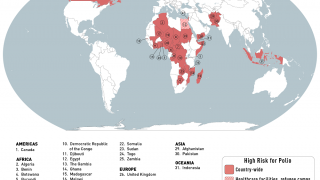
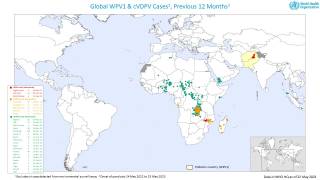
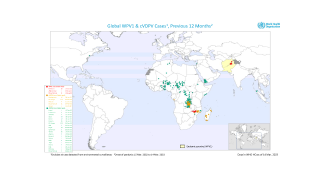
cVDPV2 outbreaks occur when the weakened poliovirus strain in the oral polio vaccine (OPV) can spread among under-immunized populations for a prolonged period and reverts to a form that can cause paralysis.
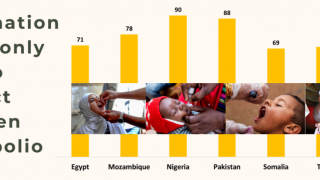
During 2019, there were 366 cases of cVDPV2 globally, while in the first 10 months of 2020 alone, there have been 588 cases.
In addition to the WHO’s Executive Board decision, the Strategic Advisory Group of Experts (SAGE) on Immunization endorsed in principle an initial use criteria framework to support early, targeted nOPV2 rollout.
Following its October 2020 meeting, the SAGE also endorsed, in principle, that nOPV2 become the vaccine of choice in response to cVDPV2 outbreaks after review of the initial use period is completed, and all requirements for nOPV2’s use are met.
Clinical studies on nOPV2, conducted in Belgium and Panama, have shown the vaccine to be safe and efficacious in protecting against polio while carrying less risk of reverting into a form that can cause paralysis under-immunized populations.
Data from these studies have been subject to WHO’s analysis for the past 6-months to determine if nOPV2 meets EUL requirements.
As detailed in the SAGE-endorsed framework, the initial use period will last for approximately 3-months following the first use of nOPV2 under EUL and will see nOPV2 deployed in a measured way to tackle ongoing outbreaks of cVDPV2.
Switzerland based Global Polio Eradication Initiative (GPEI) works closely with countries affected by cVDPV2 outbreaks to determine where nOPV2 can be used during the initial period. Factors that will inform this decision include the current epidemiology and the country’s ability to conduct the required enhanced monitoring of nOPV2’s safety and effectiveness during rollout.
Importantly, any decision to use nOPV2 will be country-led and subject to agreement from relevant in-country officials and national regulatory authorities. mOPV2 will remain available for outbreak response in countries that do not meet initial use criteria or choose not to use nOPV2 initially.
Data on nOPV2 will continue to be collected during the initial use period, in addition to further nOPV2 studies that are underway and will be conducted shortly.
Alongside nOPV2’s initial use, the GPEI will continue to implement the other strands of its comprehensive strategy to control cVDPV2 outbreaks. This includes optimizing outbreak response using mOPV2, strengthening routine immunization with inactivated polio vaccine in high-risk areas, and ensuring adequate supplies of OPV are available to reach every child.
There are other polio vaccines in development.
The nOPV2M4a polio vaccine candidate is derived from the live, infectious virus, but it has been ‘triple-locked’ using genetic engineering to prevent it from becoming harmful. nOPV2M4a is genetically more stable than existing OPVs, with a lower risk of reversion to neurovirulence.
Since 2000, the inactivated polio vaccine (IPV) is the only polio vaccine offered in the USA, says the U.S. CDC.
The IPOL vaccine is a highly purified, inactivated poliovirus vaccine with enhanced potency administered intramuscularly or subcutaneously. IPOL is a sterile suspension of three types of poliovirus: Type 1 (Mahoney), Type 2 (MEF-1), and Type 3 (Saukett).
PrecisionVaccinations publishes research-based vaccine news.
Our Trust Standards: Medical Advisory Committee