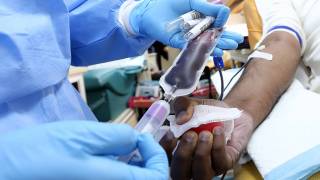
mRNA COVID-19 Vaccine Antibody Test Results Require Professional Interpretation

The U.S. Food and Drug Administration (FDA) issued a Safety Communication today reminding consumers and health care providers that currently authorized SARS-CoV-2 antibody tests should not be used to evaluate a person’s level of immunity or protection from COVID-19.
SARS-CoV-2 antibody tests help identify whether someone has antibodies to SARS-CoV-2, the beta coronavirus that causes COVID-19, indicating a prior virus infection.
The FDA stated on May 19, 2021, these antibody test results can be misinterpreted, especially after the person received a COVID-19 vaccination.
Moreover, if antibody test results are misinterpreted, there is a potential risk that people may take fewer precautions against SARS-CoV-2 exposure.
While a positive antibody test result can be used to help identify people who may have had prior SARS-CoV-2 infections, more research is needed in people who have received a COVID-19 vaccination.
The FDA says, ‘Be aware that vaccines trigger antibodies to specific viral protein targets. For example, currently authorized COVID-19 mRNA vaccines (BioNTech-Pfizer; Moderna) induce antibodies to the spike protein and not to nucleocapsid proteins that are likely detected only after natural infections.’
mRNA vaccines are considered experimental and are Authorized, but not Approved, by the FDA, as of May 19, 2021.
‘Therefore, COVID-19 vaccinated people who have not had previous natural infection will receive a negative antibody test result if the antibody test does not detect the antibodies induced by the specific type of COVID-19 vaccine.’
Currently authorized SARS-CoV-2 antibody tests have not been evaluated to assess the level of protection provided by an immune response to COVID-19 vaccination.
If you have not been vaccinated with an Authorized COVID-19 vaccine, be aware that a positive result from an antibody test does not mean you have a specific amount of immunity or protection from SARS-CoV-2 infection.
If you have a positive test result on a SARS-CoV-2 antibody test, it means that it is possible you were previously infected with the SARS-CoV-2 virus. And you should speak with your health care provider about the meaning of your SARS-CoV-2 antibody test results.
For Health Care Providers, the FDA says, ‘At this time, do not interpret the results of qualitative, semi-quantitative, or quantitative SARS-CoV-2 antibody tests as an indication of a specific level of immunity or protection from SARS-CoV-2 infection after the person has received a COVID-19 vaccination.’
‘While a positive antibody test can indicate an immune response has occurred (seroconversion), and failure to detect such a response may suggest a lack of immune response, and more research is needed.’
Currently, FDA authorized SARS-CoV-2 antibody tests are not validated to evaluate specific immunity or protection from SARS-CoV-2 infection.
For more information about antibody tests for SARS-CoV-2, see Serology/Antibody Tests: FAQs on Testing for SARS-CoV-2.
If you consider antibody testing in vaccinated individuals, follow the Centers for Disease Control and Prevention’s guidelines for antibody testing. For more information about antibody test performance, visit EUA Authorized Serology Test Performance.
Antibodies are proteins created by your body’s immune system soon after you have been infected or vaccinated. The SARS-CoV-2 antibody or serology tests look for antibodies in a blood sample to determine if an individual has had a past infection with the virus that causes COVID-19, says the FDA.
These types of tests cannot be used to diagnose a current infection.
For more information about antibody testing, see Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers.
The FDA will continue to monitor the use of authorized SARS-CoV-2 antibody tests for purposes other than identifying people with an immune response to SARS-CoV-2 from a recent or prior infection.
If you think you had a problem with a SARS-CoV-2 antibody test, the FDA encourages you to report the issue through the MedWatch Voluntary Reporting Form.
PrecisionVaccinations publishes research-based news.
Our Trust Standards: Medical Advisory Committee
- Antibody Testing Is Not Currently Recommended to Assess Immunity After COVID-19 Vaccination: FDA Safety Communication
- Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers
- CDC: Edit News Brief mRNA COVID-19 Vaccine Antibody Test Results Require Professional Interpretation | Precision Vaccinations
- Coronavirus Tests
- COVID-19 Vaccines in the USA