Mpox Research
Mpox Vaccine and Treatment Research 2024
The worldwide mpox outbreak began in May 2022 and approached a pandemic level. It has impacted various urban cities and 115 World Health Organization (WHO) member countries. To better understand the protection offered by U.S. Food and Drug Administration (FDA) Approved Jynneos® (MVA-BN) and ACAM2000 vaccines and the authorized TPOXX® (Tecovirimat) oral therapy, clinical researchers at the U.S. NIH, UKHSA, WHO, and others have published peer-reviewed insights in 2023. As of November 2023, U.S. communities are monitoring the presence of the mpox virus in wastewater, offering early warnings of mpox activity.
January 18, 2024 - Detecting Mpox Cases Through Wastewater Surveillance, US CDC - Wastewater surveillance has a sensitivity of 32% for detecting a single mpox case in wastewater samples representing thousands to millions of persons. Sensitivity increases as the number of cases in the community increases. Positive and negative predictive values are high.
January 18, 2024 - Mpox Outbreak - Los Angeles County, CA, US CDC - Mpox continues to spread within Los Angeles County. 56 cases were identified from May 4–August 17, 2023. Most mpox patients were not fully vaccinated. Two mild reinfections were reported.
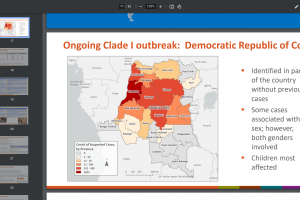
December 15, 2023 - The U.S. CDC's Morbidity and Mortality Weekly Report data indicates non-sexual, secondary mpox case rates of around 1.5%.
November 13, 2023 - An evaluation of the MPOX-SSS scoring system was published in the Journal of Infectious Diseases.
October 20, 2023 - The WHO published - Multicountry outbreak of mpox, External situation report#29 – 20 October 2023.
October 12, 2023 - The U.S. NIH confirmed that the Karius Test can detect mpox in a single vial of blood - the Potential of Pathogen-Agnostic Sequencing for Rapid Identification of Emerging Pathogens.
October 11, 2023 - A comparative analysis of Mpox neutralizing antibodies at six months from mpox infection or MVA-BN vaccination.
September 8, 2023 - The Journal of Infectious Diseases published a study: Mpox-related ophthalmic disease: a retrospective observational study in a single center in Mexico. Out of 100 cases of confirmed mpox, 11 (11%) had ocular manifestations; of these, 81.8% were people with HIV.
September 4, 2023 - The U.S. CDC published a study from The Lancet titled " The implications of MPox breakthrough infections on future vaccination strategies. " Hazra and colleagues' case series on vaccine breakthrough infections and reinfections provide valuable clinical insights into the protection against severe MPox offered by previous immunity.
September 4, 2023 - The Lancet published a study: The implications of mpox breakthrough infections on future vaccination strategies. These cases have raised concerns about a potential mpox resurgence and have underscored the need to address critical knowledge gaps concerning immunity.
September 1, 2023 - CDC MMWR - Possible Exposures Among Mpox Patients Without Reported Male-to-Male Sexual Contact — Six U.S. Jurisdictions, November 1–December 14, 2022. Among 38 (73%) patients with no known exposure to a person with mpox, behaviors preceding illness included sexual activity (17; 45%), close face-to-face contact (14; 37%), attending large social gatherings (11; 29%), and being in occupational settings (10; 26%).
August 30, 2023 - An early release October 2023 study reported that the 2022–2023 mpox outbreak predominantly affected adult men; 1.3% of reported cases were in children and adolescents <18. Analysis of global surveillance data showed one hospital intensive care unit admission and 0 deaths in that age group. Transmission routes and clinical manifestations varied across age subgroups.
August 18, 2023 - U.S. CDC - Epidemiologic and Clinical Features of Mpox in Adults Aged >50 Years — United States, May 2022–May 2023. Among 29,984 adults with mpox, those aged >50 had higher prevalences of immunocompromising conditions and HIV and a lower prevalence of symptoms than those aged ≤50.
August 18, 2023 - Study - Disparities in mpox vaccination among priority populations during the 2022 Outbreak. Conclusion: Race, insurance status, prior STI, and previous receipt of other vaccines influenced the uptake of the mpox vaccine.
July 29, 2023 - A study based on patient findings in Mexico reported proctitis was the strongest predictor of clinically confirmed mpox.
July 17, 2023 - Progress in evaluating modified vaccinia Ankara vaccine against mpox.
June 9, 2023 - U.S. CDC Notes from the Field: Exposures to Mpox Among Children Aged ≤12 Years — U.S., September 25–December 31, 2022.
June 2, 2023: The Medicine in Novel Technology and Devices journal published a study using deep learning to diagnose MPox from skin lesion images with an accuracy of over 90%.
May 26, 2023: The U.S. CDC reported substantial differences in incidence by urbanicity, gender, race, and ethnicity; 71% of cases occurred in persons residing in large urban areas. Among these mpox cases, 95.7% were in cisgender men.
May 9, 2023 - The Journal of the American Academy of Dermatology published a study that found viral DNA remained detectable in skin lesions for 17 to 31 days after symptom onset.
May 5, 2023 - The Vital Role of Research in Mpox Response: A Model for Other Diseases and Future Outbreaks.
May 5, 2023 - The JAMA Network published a Research Letter on adverse events following MVA-BN vaccination and found that local adverse event rates were highest following intradermal administration.
May 2, 2023 - The Annuals of Internal Medicine published Original Research - A Retrospective Cohort Study: Tecovirimat Treatment of People With HIV During the 2022 Mpox Outbreak. In our cohort of patients treated with tecovirimat for severe mpox, HIV status did not affect treatment outcomes.
April 28, 2023 - The U.S. CDC published Notes from the Field: Posttreatment Lesions After Tecovirimat Treatment for Mpox — New York City, August–September 2022.
On April 27, 2023, Eurosurveillence published a retrospective study on the Mpox outbreak in the Netherlands: no evidence for undetected transmission before May 2022.
April 25, 2023 - The journal Cell Host & Microbe published a study showing individuals born before 1976 and vaccinated with a smallpox vaccine had robust effector memory MPXV-specific T cell responses in mild mpox and long-lived TCF-1+ VACV/MPXV-specific CD8+ T cells decades after smallpox vaccination.
April 25, 2023 - The U.S. CDC published: Characteristics of Mpox Infections among People Experiencing Homelessness. About 60% of mpox case patients were living with HIV in LA, 49% of them virally suppressed. Hospitalization was required for 21% of case patients because of severe disease.
April 21, 2023 - The Lancet Infectious Disease published: Persistent ocular mpox infection in an immunocompetent individual over eight months, who was poorly responsive to multiple tecovirimat cycles but was successfully administered a combined antiviral treatment of tecovirimat and cidofovir.
April 6, 2023 - The Lancet reported two individuals with potential monkeypox virus reinfection at San Raffaele Hospital, Milan, Italy.
March 30, 2023 - Public Health France published an update regarding ten cases of mpox in previously vaccinated men located in the Centre-Val de Loire region, southwest of Paris.
March 24, 2023 - The Lancet published: Second clinical episode of hMPX virus in a man having sex with men. 'We believe our patient was reinfected with a different hMPX.'
March 13, 2023 - The Lancet Infectious Disease published an analysis that found using the Jynneos vaccine highly effective. Studies suggest that a single dose as PreP is preferable post-exposure prophylaxis to guarantee protection against symptomatic mpox.
February 21, 2023 - The Lancet published: Clade IIb A.3 mpox virus: an imported lineage during a large global outbreak. Evidence suggests mutations in clade IIb that are faster than expected, especially in APOBEC3 enzyme editing. In addition, other countries have reported different lineages associated with travel (A.2.1, A.2.2, and now A.3) that phylogenetically originate in Nigeria, which suggests sustained transmission in non-MSM groups.
February 21, 2023 - The Lancet published: Mpox in people with advanced HIV infection: a global case series. People living with HIV have accounted for 38–50% of those affected in the 2022 multicountry mpox outbreak. Interpretation - A severe necrotizing form of mpox in the context of advanced immunosuppression appears to behave like an AIDS-defining condition, with a high prevalence of fulminant dermatological and systemic manifestations and death.
February 20, 2023 - Possible Mpox Protection from Smallpox Vaccine–Generated Antibodies among Older Adults.
February 14, 2023 - Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model - demonstrate that the clades exhibit highly significant differences in CAST/EiJ mice in the order clade I > clade IIa > clade IIb similar to the severity of clinical disease in humans.
February 2, 2023 - The Lancet published: Treatment and prevention of mpox in pregnant women and young children.
January 31, 2023 - The Annal of Internal Medicine published Original Research: Planning for Mpox on a College Campus: A Model-Based Decision-Support Tool- This model-based analysis suggests that future outbreaks of mpox on college campuses may be controlled with timely detection and isolation of symptomatic cases.
January 25, 2023 - The U.S. CDC Volume 29, Number 4 - Research Letter: Mpox in Young Woman with No Epidemiologic Risk Factors, Massachusetts, USA. We describe a case of mpox characterized by a circularly distributed facial rash but no identified risk factors. Fomite transmission of the mpox virus from contaminated linen at a massage spa was suspected. Clinicians should consider mpox in patients with consistent clinical syndromes.
January 17, 2023 - ScienceDirect published: Development of a rapid image-based high-content imaging screening assay to evaluate therapeutic antibodies against the mpox virus. This rapid, high-content method utilizing state-of-the-art technologies enabled the evaluation of hundreds of mAbs quickly to identify several potent anti-MPXV neutralizing mAbs for further development.
January 6, 2023 - The CDC MMWR disclosed 769 mpox cases among cisgender women who confirmed recent sexual activity with men; 23 cases among pregnant or recently pregnant women were reported, and all recovered. Four pregnant women were hospitalized for mpox and administered tecovirimat, which was tolerated with no adverse reactions.
January 3, 2023 - BMJ Journals published a study that reported 10,068 individuals who received their first MVA-BN vaccination, and 15 (0.15%) developed mpox subsequently. Although the clinical presentation was similar to unvaccinated cohorts, we observed low mpox cases following a first dose of MVA-BN vaccination.
December 30, 2022 - The CDC MMWR reported that analysis of mpox infections among unvaccinated persons and those who had received 1 JYNNEOS vaccine dose ≥14 days before illness onset found that the odds of fever, headache, malaise, myalgia, and chills were significantly lower among vaccinated patients than among unvaccinated patients. Overall, 2% of vaccinated persons with mpox and 8% of unvaccinated patients were hospitalized.
December 22, 2022 - Is Mpox an STI? Why narrowing the scope of this disease may be harmful.
December 16, 2022 - A study published in the International Journal of Infectious Diseases found of the 26 Mpox patients (23%) who were given a diagnosis of bacterial tonsillitis, 6 (23%) primary syphilis, 5 (19.2%) oral or genital herpes, and 4 (15.3%) bacterial proctitis or anal abscess. The average interval between missed and correct diagnosis was 4.4 days.
December 12, 2022 - The Lancet Infectious Diseases published a study based on 77 mpox patients that the time from symptom onset to viral clearance for 90% of cases was likely 41 days in skin lesions and 39 days in semen. In immunocompetent patients with mild monkeypox disease, PCR data alone would suggest a contact isolation period of 3 to 6 weeks. However, this time could be reduced based on replication-competent virus detection.
December 8, 2022 - A descriptive case series reporting three cases of myocarditis that occurred in mpox-infected patients in France in 2022. These cases suggest an association between mpox infections and cardiac inflammatory complications. The development of chest pain in an infected patient should not be underestimated and should lead to prompt investigations for myocarditis.
December 8, 2022 - The CDC published Preliminary JYNNEOS Vaccine Effectiveness Estimates Against Medically Attended Mpox Disease in the U.S., August 15, 2022 – October 29, 2022.
December 1, 2022 - Eurosurveillance published a case-series analysis: Severe Mpox in five patients after recent vaccination with MVA-BN vaccine, Belgium, July to October 2022.
December 1, 2022 - Eurosurveillance published a study on mpox infections in 158 women (2.1%) in Spain diagnosed between April 26 and November 21, 2022. Close contact during sexual relationships was the most likely transmission mechanism. The mpox vaccination status of the women was not reported.
November 17, 2022 - The Lancet published: Human monkeypox virus infection in women and non-binary individuals during the 2022 outbreaks: a global case series.
November 2, 2022 - The BMJ published research: Transmission dynamics of monkeypox in the United Kingdom: contact tracing study.
October 31, 2022 - The CDC published Clinical Considerations for Treatment and Prophylaxis of MPV Infection in People with HIV.
October 27, 2022 - U.S. CDC Clinician Outreach and Communication Activity Call - Update on Monkeypox in Children, Adolescents, and People Who are Pregnant or Breastfeeding.
October 26, 2022 - The U.S. CDC published Severe Monkeypox in Hospitalized Patients — United States, August 10–October 10, 2022. Monkeypox was a cause of death or contributing factor in five patients, with several other deaths still under investigation.
October 17, 2022 - Researchers with the U.S. Army Medical Research Institute of Infectious Diseases detect monkeypox virus in the testes of nonhuman primate survivors.
October 17, 2022 - The U.S. CDC published an Early Release MMWR: Ocular Monkeypox — United States, July–September 2022. This report describes five cases of ocular monkeypox identified in the United States during July–September 2022. Patients with ocular monkeypox, including those with HIV-associated immunocompromise, have experienced delays in treatment initiation, prolonged illness, hospitalization, and vision impairment.
October 17, 2022 - The U.S. CDC reported - Monkeypox Virus Infection Resulting from an Occupational Needlestick — Florida, 2022. This report describes the first occupationally acquired MPVX infection in a U.S. healthcare worker during the 2022 monkeypox outbreak.
October 12, 2022 - The NEJM published a CORRESPONDENCE: Neonatal Monkeypox Virus Infection. Reports of neonatal monkeypox virus infection are rare.3 This was a neonatal monkeypox virus infection after peripartum transmission within a family cluster; transplacental transmission could not be ruled out.4 Because this was a single case, it is not possible to attribute the clinical illness to either pathogen (monkeypox virus or adenovirus) directly, nor is it possible to attribute the improvement in the infant's clinical condition to the use of tecovirimat or cidofovir.
October 7, 2022 - The Lancet Infectious Diseases published a study showing that the monkeypox outbreak is primarily fueled by sexual contact.
October 7, 2022 - The Lancet Microbe published a study - Air and surface sampling for monkeypox virus in a U.K. hospital: an observational study - These data show contamination in isolation facilities and the potential for suspension of MPXV into the air during specific activities. PPE contamination was observed after clinical contact and changing of bedding. Contamination of hard surfaces in doffing areas supports the importance of cleaning protocols, PPE use, and doffing procedures.
October 6, 2022: The U.S. CDC clinician outreach and communication activity call will provide a situational update for clinicians about severe monkeypox virus infections.
October 5, 2022 - The peer-reviewed journal Nature published: The monkeypox virus is mutating. Are scientists worried?
September 29, 2022 - The Lancet published: Viral loads in clinical samples of men with monkeypox virus infection: a French case series. High MPXV viral loads from skin and mucosa, including genital and anal sites, suggest that transmission most likely occurs through direct body contact rather than through the respiratory route or contact with body fluids, which should help to refine the prevention messages delivered to individuals most exposed to the virus.
September 29, 2022 - The U.S. CDC issued CDCHAN-00475 - Severe Manifestations of Monkeypox among PImmunocompromised People to HIV or Other Conditions.
September 23, 2022 - The UKHSA published " Research and analysis - Investigation into monkeypox outbreak in England: technical briefing #8. "
September 20, 2022 - Is there any evidence for milder courses of monkeypox virus infections with childhood smallpox vaccination? Our results may provide limited evidence for a more favorable disease course with a history of smallpox vaccination. However, hospitalization rates were not lower in vaccinated persons.
September 19, 2022 - An Early Release report published by the U.S. CDC: Monkeypox in a Young Infant — Florida, 2022.
September 17, 2022 - The Indian Council of Medical Research- NIV Pune analyzed the complete genome sequences of Monkeypox cases and found three sub-clusters among A.2 lineage – first cluster Kerala (n5) and Delhi (n2) aligned with the USA-2022 ON674051.1; while the second of Delhi (n3) aligned with USA-2022 ON675438.1 and third cluster consists of the U.K., U.S., and Thailand.
September 16, 2022 - The U.S. CDC published " Health Care Personnel Exposures to Subsequently Laboratory-Confirmed Monkeypox Patients — Colorado, 2022 ".
September 13, 2022 - The U.S. CDC's MMWR - Two Cases of Monkeypox-Associated Encephalomyelitis — Colorado and the District of Columbia, July–August 2022. Two U.S. cases of encephalomyelitis associated with acute MPXV infection were identified in the summer of 2022, whether the underlying pathophysiology resulted from direct viral neuroinvasion or a parainfectious autoimmune process.
September 9, 2022 - The U.S. CDC published " Clinical Use of Tecovirimat (Tpoxx) for Treatment of Monkeypox Under an Investigational New Drug Protocol. " Human data demonstrate the efficacy of tecovirimat, and clinical trials are necessary to elucidate its clinical efficacy in patients with monkeypox virus infection, indications for treatment, and the ideal duration of treatment.
September 9, 2022 - The U.S. CDC's Morbidity and Mortality Weekly Report - HIV and Sexually Transmitted Infections Among Persons with Monkeypox — Eight U.S. Jurisdictions, May 17–July 22, 2022.
September 4, 2022 - The journal HIV Research published an Original Article: Clinical characteristics of monkeypox virus infections among men with and without HIV: A large outbreak cohort in Germany. In this preliminary cohort analysis from a current large outbreak among MSM in Germany, the clinical picture of MPXV infection did not differ between MSM with and without HIV infection. However, most patients were relatively healthy, and only a few people living with HIV were viremic or severely immunosuppressed.
September 1, 2022 - The U.S. CDC issued Technical Report #2 - Multi-National Monkeypox Outbreak, United States. Tecovirimat is generally well tolerated, and these data support continued access to treatment with tecovirimat during the current monkeypox outbreak.
September 1, 2022 - A non-peer-reviewed, limited clinical study conducted in The Netherlands concluded that 'a primary MVA-BN immunization series in non-primed individuals yields relatively low levels of MPXV neutralizing antibodies.'
August 19, 2022 - The Journal of Infection published a Letter: First case of monkeypox virus, SARS-CoV-2, and HIV co-infection.
August 19, 2022 - The UKHSA published research and analysis - Investigation into monkeypox outbreak in England: technical briefing #6.
August 16, 2022 - The U.S. CDC published Volume 28, Number 10—October 2022: Research Letter - Human Monkeypox without Viral Prodrome or Sexual Exposure, California, USA, 2022. Abstract: We report human monkeypox in a man who returned to the United States from the United Kingdom and reported no sexual contact. He had vesicular and pustular skin lesions but no anogenital involvement. The potential modes of transmission may have implications for the risk of spread and epidemic control.
August 16, 2022 - The Annals of Internal Medicine published: Detection of Monkeypox Virus in Anorectal Swabs From Asymptomatic in a Sexually Transmitted Infection Screening Program in Paris, France. Summary: the practice of ring postexposure vaccination around symptomatic persons with probable or confirmed MPXV infection may not be sufficient to contain the virus spread.
August 12, 2022 - The peer-reviewed journal Nature Medicine published: Retrospective detection of asymptomatic monkeypox virus infections in Belgium. These findings show that some instances of monkeypox remain undiagnosed and suggest that testing and quarantining individuals reporting symptoms may not suffice to contain the outbreak.
August 10, 2022 - The Lancet published the first documented genomic sequencing case of human-to-dog (greyhound) monkeypox transmission in France.
August 9, 2022 - Peter Marks, MD, Ph.D., U.S. FDA, published EUA Application Number 28801: Assessment of two doses (each 0.1 mL dose containing 2 x 107 TCID50 of MVA-BN) of JYNNEOS via the intradermal (I.D.) route of administration for prevention of monkeypox disease in individuals 18 years of age and older determined to be at high risk for monkeypox infection and the use of two-doses (each 0.5 mL dose containing 1 x 108 TCID50 of MVA-BN) of JYNNEOS via the subcutaneous (S.C.) route of administration for prevention of monkeypox disease in individuals under 18 years of age determined to be at high risk for monkeypox infection.
August 6, 2022 - The Hindu published a study that revealed that the rate of genetic changes in the monkeypox virus was higher than expected and varied by country.
August 5, 2022 - The U.S. CDC Early-Release MMWR: Interim Guidance for Prevention and Treatment of Monkeypox in Persons with HIV Infection — United States, August 2022. The CDC developed clinical considerations for preventing and treating MPX in persons with HIV infection, including pre-exposure and post-exposure prophylaxis with the JYNNEOS vaccine, treatment with tecovirimat, and infection control.
August 5, 2022 - The U.S. CDC published: Epidemiologic and Clinical Characteristics of Monkeypox Cases — United States, May 17–July 22, 2022. These data can guide clinical considerations for evaluating persons for monkeypox. Typically, monkeypox begins with a febrile prodrome, which might include malaise, chills, headache, or lymphadenopathy, followed by a disseminated rash that often consists of the palms and soles. Although 42% of persons did not report prodromal symptoms, and 37% did not report fever by the time of the interview. Genital rash, although reported in fewer than 50% of cases, was common; 36% developed a rash in four or more body regions.
August 2, 2022 - The Lancet published Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. The case discussed herein supports that transmission of the monkeypox virus during sexual activity might be a viable and recognized route.
July 28, 2022 - The BMJ published Research: Clinical features and novel presentations of human monkeypox in a central London center during the 2022 outbreak: descriptive case series. A variable temporal association between mucocutaneous and systemic features was observed, suggesting a new clinical course to the disease. Technical presentations of monkeypox infection were also identified, including rectal pain and penile edema.
July 25, 2022 - This UKHSA guidance describes case definitions to inform testing and reporting suspected monkeypox cases.
July 23, 2022 - MIT Tech Review reported that Stanford's Sewer Coronavirus Alert Network added monkeypox to the viruses it checks wastewater for. Monkeypox has been detected in 10 of the 11 sewer systems SCAN tests, including those in Sacramento, Palo Alto, and several other cities in California's Bay Area. On July 22, 2022, SFDPH reported the total number of MPX cases in San Francisco residents to 197.
July 22, 2022 - The UKHSA published: Research and analysis - Investigation into monkeypox outbreak in England: technical briefing #4.
July 21, 2022 - The NEJm published an ORIGINAL ARTICLE: Monkeypox Virus Infection in Humans across 16 Countries — April–June 2022. In this case series, monkeypox manifested with dermatologic and systemic clinical findings.
July 21, 2022 - Euroserveilance published a Rapid communication - Paediatric monkeypox patient with an unknown infection source in the Netherlands in late June 2022. We were not able to identify any possible source of the infection. Whole genome sequencing positioned the patient's sequence within the clade three lineages B.1 but did not directly link to other strains from the Amsterdam region.
July 14, 2022 - Eurosurveillance published: Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. 'Here, we find that MPX DNA was detected in saliva at some point in all 12 patients studied, in the samples collected between 4–16 days after the onset of symptoms. With the low Cq values observed in our study in a variety of samples such as saliva, rectal swabs, semen, urine, and fecal samples, further research on the infectious potential of these bodily fluids and their potential role in disease transmission by close physical contact during sexual activity is warranted.'
July 8, 2022 - The UKHSA published Technical Briefing #3. There have been four female cases and one child (under 16) in England. The UKHSA monkeypox nowcasting model implies positive growth between 3.8% and 6.7% per day, corresponding to a doubling time of 15 days (90% CI: 10 days, 18 days).
July 8, 2022 - The ECDC's updated rapid risk assessment includes novel insights from a stochastic mathematical model developed collaboratively with the European Health Emergency Preparedness and Response Authority to assess vaccination strategies as outbreak response measures. Higher effectiveness of contact tracing can be achieved, and combined with high vaccine uptake levels of 80% (upper bound estimate), the chance of MPXV outbreak control by week 12 can be maximized, with PrEP vaccination being the most effective strategy.
July 5, 2022 - The U.K. Health Security Agency stated the current outbreak clade of monkeypox is no longer classified as a high-consequence infectious disease following a review by the Advisory Committee on Dangerous Pathogens (ACDP) and agreement by the U.K.'s four nations' public health agencies.
July 5, 2022 - A non-peer-reviewed article - Asymptomatic monkeypox virus infections among males in Belgium - concluded: 'The existence of asymptomatic monkeypox infection indicates that the virus might be transmitted to close contacts in the absence of symptoms.'
July 1, 2022 - The Lancet published: Demographic and clinical characteristics of confirmed human MPXV cases in individuals attending a sexual health center in London, UK: an observational analysis. Interpretation - Autochthonous community MPXV transmission is currently observed among MSM in London. We found many concomitant STIs and frequent anogenital symptoms, suggesting transmissibility through local inoculation during close skin-to-skin or mucosal contact during sexual activity. Only five patients required hospitalization, and no one died.
June 29, 2022 - The U.S. CDC's Clinical Outreach and Communication Activity presentation highlighted various technical aspects of the evolving MPXV outbreak.
June 27, 2022 - The Lancet Public Health published a Commentary from the Royal College of Obstetricians and Gynaecologists staff: Monkeypox vaccines in pregnancy: lessons must be learned from COVID-19. Data on monkeypox infection in pregnancy are minimal. However, the related orthopoxvirus, smallpox, is associated with an increased risk of maternal and perinatal morbidity and mortality, including fetal death, preterm birth, and miscarriage. Currently, no vaccine against monkeypox is approved for use in pregnancy. Whether the MVA-BN vaccine passes into breastmilk remains unknown, but this is unlikely because the vaccine virus does not replicate effectively in humans.
June 24, 2022 - The UKHSA published Technical Briefing #2 - an Investigation into the monkeypox outbreak in England (London). The MPXV strain in the U.K. contains 48 single mutations in its genome relative to 2018. Twenty-seven of these mutations are silent, and twenty-one cause changes in viral proteins.
June 24, 2022 - Researchers from Portugal's National Institute of Health in Lisbon published a study in the peer-reviewed journal Nature showing that this MPXV belongs to clade three and that the outbreak most likely has a single origin. Although 2022 MPXV (lineage B.1) clustered with 2018–2019 cases linked to an endemic country, it segregates in a divergent phylogenetic branch, likely reflecting continuous accelerated evolution.
June 24, 2022 - A genetic analysis published in the peer-reviewed journal Nature Medicine revealed that the virus microevolution responsible for the monkeypox outbreak in 2022 might be related to viruses responsible for human cases in the U.K., Israel, and Singapore in 2018 and 2019.
June 23, 2022 - The Lancet published a modeling study: Projected burden and duration of the 2022 Monkeypox outbreaks in non-endemic countries. 'In conclusion, our findings align with WHO's assessment that the overall public health risk at a global level is moderate. Observed outbreaks in non-endemic countries should be contained quickly, mainly when adequate mitigation measures are implemented. The two intervention scenarios showed that interventions targeting contacts of primary cases could reduce the median duration of monkeypox outbreaks by between 60·9% and 75·7%.
June 9, 2022 - The Lancet published an Editorial: Monkeypox: a neglected old foe. African populations have coexisted with the MPXV for decades, and it is time for research to address the needs of endemic countries. A study on the impact of smallpox vaccination on monkeypox cases among healthcare workers is ongoing in DR Congo. Another study will monitor expanded access to tecovirimat (TPOXX), the only antiviral drug licensed to treat monkeypox in the Central African Republic.
May 24, 2022 - The peer-reviewed journal The Lancet Infectious Disease published: Clinical features and management of human monkeypox: a retrospective observational study in the U.K. Interpretation - Human monkeypox poses unique challenges, even to well-resourced healthcare systems. Prolonged upper respiratory tract viral DNA shedding after skin lesion resolution challenged current infection prevention (Jynneos) and control guidance (TPOXX).
November 14, 2019 - The peer-reviewed journal NEJM published an Original Article in which researchers found that 14 days after a single MVA vaccination, the levels of antibodies that neutralize the monkeypox peaked at a level similar to ACAM2000. Given that the vaccines are presumed to work postexposure if given within two weeks of virus contact, this finding suggests a single dose of MVA could prevent many MPX cases. CONCLUSIONS: No safety concerns associated with the MVA vaccine were identified. Immune responses and attenuation of the primary cutaneous reaction suggest that this MVA vaccine protects against variola infection.
September 4, 2018 - The peer-reviewed journal Frontier in Public Health published " Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. " The frequency and geographical spread of human monkeypox cases have increased in recent years, and there are considerable gaps in our understanding of the disease's emergence, epidemiology, and ecology. As a result, the monkeypox virus, considered a high-threat pathogen, is causing a disease of public health importance.
June 21, 2016 - PLOS One published: A Randomized, Double-Blind, Placebo-Controlled Phase II Clinical Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN®) in 56-80-Year-Old Subjects. The results suggest that a single dose of MVA in a 56–80-year-old population was well tolerated and sufficient to rapidly boost the long-term B cell memory response induced by a prior vaccination with a traditional smallpox vaccine.
May 5, 2015 - Open Forum Infectious Disease published: Safety and Immunogenicity of Modified Vaccinia Ankara-Bavarian Nordic Smallpox Vaccine in Vaccinia-Naive and Experienced Human Immunodeficiency Virus-Infected Individuals: An Open-Label, Controlled Clinical Phase II Trial. Conclusions - MVA was safe and immunogenic in subjects infected with HIV and represents a promising smallpox vaccine candidate for use in immunocompromised populations.
March 20, 2015 - PLOS One published a Research Article: A Prospective Study of the Incidence of Myocarditis/Pericarditis and New Onset Cardiac SymptoFollowinging Smallpox and Influenza Vaccination. Conclusions - Passive surveillance significantly underestimates the true incidence of myocarditis/pericarditis after smallpox immunization. In addition, evidence of subclinical transient cardiac muscle injury post-vaccinia immunization is a finding that requires further study to include surveillance of long-term outcomes.
January 17, 2014 - U.S. CDC: Volume 20, Number 2—February 2014 - Research: Genomic Variability of Monkeypox Virus among Humans, the Democratic Republic of the Congo.
March 12, 2013 - The Journal of Infectious Diseases published: A randomized, controlled clinical trial: Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients. Conclusions - MVA-BN is safe, well-tolerated, and immunogenic in HSCT recipients. These data support the use of 108 TCID50 of MVA-BN in this population.
August 30, 2010 - PNAS published: There has been a significant increase in human monkeypox incidence 30 years after smallpox vaccination campaigns ceased in the DRCongo. A comparison of active surveillance data in the same health zone from the 1980s (0.72 per 10,000) and 2006–07 (14.42 per 10,000) suggests a 20-fold increase in human monkeypox incidence. Thirty years after mass smallpox vaccination campaigns ceased, human monkeypox incidence has dramatically increased in rural DRC.
May 22, 2008 - PNAS published a peer-reviewed study using two challenge doses of MPXV administered intravenously with one dose of Dryvax (ACAM2000) manufactured by Wyeth Laboratories, Inc. Researchers found that a single injection of MVA within four days of exposure induces rapid protection in the MPX model, which suggests MVA might be effective with unimmunized individuals during a smallpox outbreak.
Mpox Virus Genomic Epidemiology
The AfriAfrican reported the initial human infection caused by the MPXV was identified in Basankusu Territory, the Democratic Republic of the Congo, in 1970. The 2022 MPXV diverges from the related 2018–2019 viruses by a mean of 50 single-nucleotide polymorphisms, which is far more (roughly 6–12-fold more) than one would expect considering previous estimates of the substitution rate for Orthopoxviruses (1–2 substitutions per genome per year). In 2014, a study - Genomic Variability of MPXV among Humans, DRC - detected four distinct lineages and a deletion resulting in gene loss in 10 (16.7%) samples that seemed to correlate with human-to-human transmission (p = 0.0544).
This data repository contains updates and details about MPXV lineage designations. These lineages only apply to recent human cases and are distinct from broader MPXV clad circulating in animal reservoirs I, IIa, and IIb. For example, the recent mpox outbreak among humans originated from clade IIb.
Mpox Clades
Mpox virus phylogeny shows two distinct clusters corresponding to the previously recognized clades. The Congo Basin clade became Clade I, and the West African clade became Clade II, encompassing two phylogenetically different subclades, IIa and IIb. Neither subclade is descended from the other. Furthermore, there are appreciable genetic differences between Clades I and II, showing nearly twice the divergence between subclades IIa and IIb. Nonetheless, both subclades include genomes from the 1960s and 1970s and appear to have evolved separately.