Malaria Vaccine Mosquirix Protects Over the Long-Term

A large phase 3 clinical trial of the Mosquirix (RTS,S/AS01) vaccine delivered long-term protection from malaria, a mosquito-borne disease caused by a parasite.
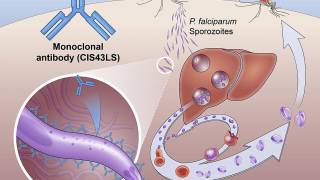
Mosquirix is a recombinant malaria preventive vaccine consisting of the P. falciparum circumsporozoite protein from the pre-erythrocytic stage.
The Mosquirix vaccine aims to trigger the human immune system to defend against the first stages of malaria when the P. falciparum parasite enters the host’s bloodstream through a mosquito bite and infects liver cells.
Overall, this large 3-year extension phase 3 clinical trial of 1,739 older children (aged 5–7 years) and 1,345 younger children (aged 3–5 years) found severe malaria incidence was low in all study groups.
Furthermore, there was no evidence of malaria recurrence in those who received Mosquirix vaccination, despite an increased incidence of clinical malaria in older children.
This follow-on study did not report vaccine-related severe adverse events. The initial Phase 3 trial of Mosquirix was conducted over 5 years (2009–2014).
This is good news since more than 219 million new malaria cases were reported in 87 countries in 2018, says the World Health Organization (WHO).
According to WHO’s latest World malaria report, the number of countries with less than 100 indigenous cases of malaria – a strong indicator that elimination is within reach – increased from 15 in 2010 to 26 in 2017.
Since 2018, 4 countries have been certified by WHO as malaria-free: Algeria, Argentina, Paraguay, and Uzbekistan.
About 1,700 cases of malaria are diagnosed in the USA annually, mostly in returning international travelers.
The WHO recently published its World Malaria Report 2018, which unfortunately highlight that ‘no significant progress in reducing global malaria cases was made in 2016-2017'.
WHO insights
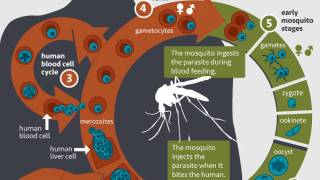
- Malaria is caused by Plasmodium parasites that are spread to people through the bites of infected Anopheles mosquito vectors. Of the 5 parasite species that cause malaria in humans, P. falciparum is the most deadly. The first symptoms of malaria – fever, headache, and chills – usually appear 10–15 days after the infective mosquito bite. Left untreated, P. falciparum malaria can progress to severe illness and death.
- Pregnant women are also at considerably higher risk of contracting malaria, and developing severe disease, than other populations. Malaria in pregnancy increases the risk of maternal and fetal anemia, stillbirth, spontaneous abortion, low birth weight, and infant death.
- Early diagnosis and treatment of malaria reduces disease and prevents deaths. It also contributes to reducing malaria transmission. Access to diagnostic testing and treatment should be seen not only as a component of malaria control but as a fundamental right of all populations at risk.
The US Centers for Disease Control and Prevention (CDC) publishes various travel alerts since millions of US residents travel to countries where malaria is present.
Travel vaccine and medication counseling sessions can be scheduled with local pharmacies at Vax-Before-Travel.
As of July 1, 2019, the CDC updated its private sector vaccine prices for general information.
And, the CDC’s Vaccines For Children program offers vaccines at no cost to children who might not otherwise be vaccinated because of inability to pay.
Additional financial support programs can be found at Vaccine Discounts.
Vaccines, like any medicine, can have side effects. You are encouraged to report vaccine side effects to the CDC.
This study was published in The Lancet and was funded by GlaxoSmithKline Biologicals.
Our Trust Standards: Medical Advisory Committee