Mpox Testing April 2023
Mpox Testing April 2023
The Mpox virus is a viral zoonosis diagnosed by PCR test on a viral swab taken from one or more vesicles or ulcers, says the U.K. Health Security Agency (UKHSA). For high-risk contacts of a confirmed case who have developed systemic symptoms but do not have a rash or lesions for sampling, you should take a throat swab in viral transport media, recommends the World Health Organization (WHO).
The U.S. Administration updated as of April 6, 2023, non-variola orthopoxvirus/mpox testing from public health and select commercial laboratories.
As of April 5, 2023, communities in the U.S. can monitor the presence of the mpox virus in wastewater samples. Data from samples can provide an early warning of mpox activity and spread in communities. One community has had consistent mpox detection in the past four weeks.
Mpox Test Method
The Mpox virus (MPXV) is part of the same family of viruses as smallpox and can be detected by diagnostic testing. The U.S. CDC recommends that clinicians collect two specimens for each patient, each from multiple lesions, preferably from different locations on the body and from lesions with differing appearances. The CDC testing algorithm includes non-variola Orthopoxvirus testing, and if results are positive for Orthopoxvirus, the CDC should complete further characterization testing. And Orthopoxvirus PCR will only be used when the Mpox PCR assay results require further clarification.
On October 7, 2022, the U.S. FDA issued an Emergency Use Authorization (EUA) to Abbott Molecular, Inc., for the Alinity m MPXV, a real-time PCR test intended to detect monkeypox DNA using lesion swab specimens from individuals suspected of MPXV infection. In addition, on September 9, 2022, the FDA published answers to FAQs relating to the development and performance of tests for Mpox.
And the FDA announced on August 12, 2022, 'Given the robustness of the existing safeguards for blood safety, the FDA does not recommend those blood establishments ask donors additional, specific questions about possible exposure to monkeypox virus. And on July 15, 2022: "The FDA is not aware of clinical data supporting the use of other sample types, such as blood or saliva, for monkeypox virus testing." On July 14, 2022, Eurosurveillance published a study that found the DNA of the poxvirus in semen, saliva, urine, and feces.
Mpox Current Procedural Terminology
The new AMA-issued laboratory test CPT code (87593) describes molecular diagnostic testing that detects the nucleic signature of an orthopoxvirus, including the monkeypox virus.
Mpox Test News
January 9, 2023 - Becton, Dickinson, and Company and CerTest Biotec announced EUA from the U.S. FDA for a molecular polymerase chain reaction assay for Mpox virus detection. The VIASURE Mpox Virus Real-Time PCR Reagents for BD MAX System is now available for BD MAX™ System users.
August 18, 2022 - New Jersey-based GenScript USA Inc. announced the immediate availability of a monkeypox virus PCR test kit, which can be ordered by diagnostic labs and medical device distributors to be used in research to provide detection of the monkeypox virus. The test kit has already received the C.E. mark for IVD use in the European Union.
August 17, 2022 - JOYSBIO announced the development of two Monkeypox tests, both of which deliver results in 15 minutes or less. JOYSBIO recently launched a Monkeypox Antigen Rapid test and a Monkeypox IgM/IgG Antibody Rapid test, both are CE-IVD marked. Both tests are currently in clinical evaluation in Europe and showed reliable results with infected patients.
August 12, 2022 - The U.S. FDA announced that 'Worldwide, there have been no reports of monkeypox virus transmission through blood transfusion, and the risk of transfusion-transmission remains theoretical. The virus levels in an infected or exposed individual's blood have not been well characterized.'
August 4, 2022 - Rochelle Walensky, MD, MPH, @CDCDirector: Tweeted Monkeypox testing has significantly increased from 500 specimens tested each week in late May to nearly 8,000 specimens tested last week. Laboratories can perform 80,000 testweeklyek, which is much more than the current demand.
July 18, 2022 - Sonic Healthcare USA began testing for Monkeypox using the U.S. CDC Non-variola Orthopoxvirus, high complexity NAAT molecular assay in Austin, TX, and is available for providers to order through all Sonic clinical laboratories located across the USA.
July 18, 2022 - HealthTrackRx is working in partnership with the U.S. CDC to conduct an epidemiologic study to help to understand how widespread Monkeypox is within the USA.
July 15, 2022 - The U.S. FDA recommends using swab samples taken directly from a lesion when testing for MPXV. The FDA is not aware of clinical data supporting the use of other sample types, such as blood or saliva, for monkeypox virus testing.
July 11, 2022 - Mayo Clinic Laboratories began testing for monkeypox using CDC's orthopoxvirus test, which detects most non-smallpox-related orthopoxviruses, including monkeypox. Mayo Clinic Laboratories can accept specimens from anywhere in the USA and expects to be able to perform up to 10,000 tests per week.
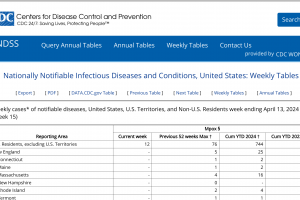
July 8, 2022 - The U.S. CDC published a Morbidity and Mortality Weekly Report - U.S. LRN laboratories validated to perform the non-variola Orthopoxvirus (NVO) assay from May 17–June 30, 2022, testing 2,009 specimens from patients with suspected MPXV. Among these, 730 (36%) specimens from 395 patients were positive for NVO.
July 6, 2022 - Labcorp began testing for monkeypox using CDC's orthopoxvirus test (which detects all non-smallpox-related orthopoxviruses, including monkeypox).
July 1, 2022 - The Africa Centre for Disease Control (ACDC) and the African Society for Laboratory Medicine jointly held their first Real-Time PCR-based Monkeypox virus testing training for twenty African Union Member States.
June 27, 2022 - The WHO confirmed any individual meeting the definition for a suspected case should be offered to test.
June 24, 2022 - The UKHSA published guidance: Monkeypox diagnostic testing. Information on taking, submitting, and processing sample-containing in the monkeypox virus.