Malaria Medicine Approved For the Radical Cure of P. Vivax Malaria

GlaxoSmithKline (GSK) and Medicines for Malaria Venture (MMV) today announced that the Food and Drug Administration (FDA) has approved the single-dose Krintafel (tafenoquine) for the radical cure (prevention of relapse) of Plasmodium vivax (P. vivax) malaria in people older than 16 years of age and who are receiving appropriate antimalarial therapy for acute P. vivax infection.
P. vivax malaria has a significant public health and economic impact, primarily in South-Asia, South-East Asia, Latin America and the Horn of Africa. The disease is estimated to cause around 8.5 million clinical infections every year.
The United States has approximately 1,700 cases of imported malaria occurring each year.
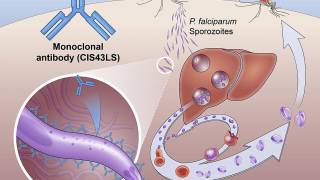
Krintafel is an 8-aminoquinoline derivative with activity against all stages of the P. vivax life cycle, including hypnozoites.
Dr. Hal Barron, Chief Scientific Officer, and President of Research and Development, GSK, said: “Today’s approval of single-dose Krintafel, the first new treatment for Plasmodium vivax malaria in over 60 years, is a significant milestone for people living with this type of relapsing malaria.
This FDA approval was based on efficacy and safety data from a comprehensive global clinical development P. vivax radical cure programme designed in agreement with the FDA.
With the approval of Krintafel, the FDA awarded GSK a tropical disease priority review voucher, which is designed to encourage the development of new drugs and biological products for prevention and treatment of certain neglected tropical diseases affecting millions of people throughout the world.
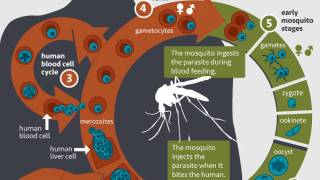
The Plasmodium parasite is a complex organism with a lifecycle spanning both humans and mosquitoes. After an infected mosquito bite, the P. vivax parasite infects the human blood and causes an acute malaria episode.
It also has the ability to lie dormant in the liver (in a form known as hypnozoite) from where it periodically reactivates to cause relapses of P. vivax malaria.
Hence, a single P. vivax infection can give rise to multiple episodes of malaria, in the absence of a new mosquito bite. These relapses can occur weeks, months or even years after the initial infection.
The dormant liver forms of the parasite cannot be readily treated with most anti-malarial treatments active against the blood-stage parasite.
The 8-aminoquinoline, primaquine, is currently the only FDA approved medicine that targets the dormant liver stage to prevent relapse. It must be taken for 14 days to be effective, a regimen that is associated with poor compliance.
The use of a medicine that targets the dormant liver forms of the parasite, co-administered with currently available antimalarials such as chloroquine or artemisinin-based combination therapies (ACTs) is known as a radical cure.
The clinical features of P. vivax malaria include fever, chills, vomiting, malaise, headache and muscle pain, and in some cases, can lead to severe malaria and be fatal.
Krintafel should not be administered to:
- patients who have glucose-6-phosphate dehydrogenase (G6PD) deficiency or have not been tested for G6PD deficiency
- patients who are breastfeeding a child known to have G6PD deficiency or one that has not been tested for G6PD deficiency
- patients who are allergic to tafenoquine or any of the ingredients in Krintafel or who have had an allergic reaction to similar medicines containing 8-aminoquinolines.
The use of Krintafel during pregnancy may cause hemolytic anemia in a G6PD-deficient fetus. Krintafel is not recommended during pregnancy.
Females of reproductive potential should avoid pregnancy or use effective contraception for 3 months after the dose of Krintafel.
Additionally, a G6PD-deficient infant may be at risk for hemolytic anemia from exposure to Krintafel through breast milk. Infant G6PD status should be checked before breastfeeding begins.
Krintafel is contraindicated in breastfeeding women when the infant is found to be G6PD deficient or the G6PD status of the infant is unknown. A woman with a G6PD-deficient infant or if the G6PD status of the infant is not known should not breastfeed for 3 months after the dose of Krintafel.
Approximately 37 percent of malaria cases in the USA occur in women, including 5–6 percent who are pregnant at the time they are infected.
When a woman is infected with malaria during pregnancy, they are at an increased risk for maternal and fetal complications, says the Centers for Disease Control and Prevention (CDC).
Treatment options for uncomplicated, chloroquine-resistant P. falciparum and P. vivax malaria infections in pregnant women are threatened by the spread of mefloquine resistance.
To address this need, the CDC published strong evidence of the safety and efficacy of using artemether-lumefantrine (AL, or Coartem) to treat malaria in the second and third trimesters of pregnancy.
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
GSK is a science-led global healthcare company. For further information please visit www.gsk.com.
Medicines for Malaria Venture (MMV) - MMV is a leading product development partnership (PDP) in the field of antimalarial drug research and development.
Our Trust Standards: Medical Advisory Committee