First-in-Human MERS Vaccine Published Positive Results

A Pennsylvania-based, clinical-stage biotechnology company announced positive results from the first-in-human, Phase 1 clinical trial of its INO-4700 vaccine against the Middle East Respiratory Syndrome Coronavirus (MERS).
This is important news since there is not a MERS-prevention vaccine nor therapeutics available, as of July 24, 2019.
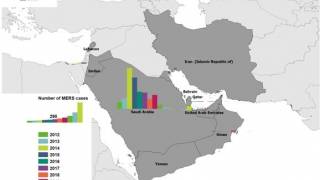
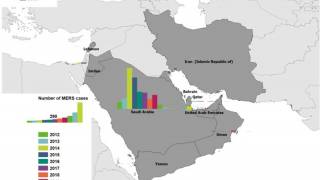
Previously, the World Health Organization (WHO) reported on May 31, 2019, this respiratory virus, now known as MERS-CoV, has infected more than 2,442 people worldwide and has been fatal in approximately 35 percent of patients to date.
This peer-reviewed article published July 2019, entitled, "Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial," reported study subjects vaccinated with INO-4700, also called GLS-5300, displayed robust levels of MERS antigen-specific antibody and T cell responses at week 14 (2-weeks post-third dose).
These vaccine-generated immune responses to INO-4700 were durable as they were maintained through 60 weeks following dosing.
Additionally, Inovio's MERS DNA vaccine was well-tolerated and demonstrated overall high levels of antibody responses in 94 percent of subjects, while also generating broad-based T cell responses in 88 percent of study participants.
Furthermore, INO-4700 administration generated antibody responses with similar potency compared to those of patients who were infected with the MERS virus and subsequently recovered from the South Korea MERS outbreak.
Even more interestingly, the vaccination generated more robust T cell responses than convalescent patients, suggesting the vaccine's ability to protect from reinfection from MERS virus, said the company in a press release.
Dr. Kayvon Modjarrad, director of WRAIR's Emerging Infectious Diseases Branch, the principal investigator of the study and first author on the publication, said in a press release, "The world witnessed the emergence and devastation of SARS in 2002 and then MERS 10 years later. MERS hasn't gone away. And there's every indication that the family of viruses to which SARS and MERS belong, coronaviruses, are here to stay.”
“U.S. military personnel are at particular risk for MERS, given the deployments to the Middle East where the largest MERS outbreaks have occurred.”
“This study is, therefore, an important advancement for the U.S. Army, the military community as a whole and the global stakeholders in the research and development of both MERS and coronavirus countermeasures."
Middle East Respiratory Syndrome is caused by a coronavirus that is related to the virus which causes severe acute respiratory syndrome (SARS), says the WHO.
While the SARS coronavirus infected and caused illness in more than 8,000 people worldwide, the disease was short-lived between 2002 and 2004 and had a case fatality rate of about 10 percent.
MERS news
- Saudi Arabia Reports 14 More MERS Coronavirus Cases
- MERS-CoV Returns to England
- Hajj Vaccination Requirements Updated for 2019
This phase 1 study was funded by the U.S. Department of the Army and GeneOne Life Science, Inc. and conducted at WRAIR. This work was partially supported through a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DoD).
GeneOne and the International Vaccine Institute have continued the clinical development efforts on this vaccine as part of a second, Phase 1b/2a trial that is currently ongoing in Korea with the interim data expected later this year. The Wistar Institute also collaborated on the development of this vaccine.
Inovio plans to initiate a larger Phase 2 field trial in the Middle East through a partnership with CEPI, based on Phase 1b/2a data for INO-4700. Inovio received a $56 million funding last year from CEPI under which Inovio will develop vaccine candidates through Phase 2 against MERS and Lassa fever.
The shared goal of Inovio and CEPI is for a MERS vaccine to be available as soon as possible for emergency use as a stockpile post-Phase 2 testing.
Inovio Pharmaceuticals, Inc. is an innovative clinical-stage biotechnology company focused on the discovery, development, and commercialization of its synthetic DNA technology targeted against cancers and infectious diseases.
Published by Vax Before Travel
Our Trust Standards: Medical Advisory Committee
- Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm
- Phase I, Open Label Dose Ranging Safety Study of GLS-5300 in Healthy Volunteers
- Inovio's Positive First-in-Human MERS Vaccine Results Published in The Lancet Infectious Diseases
- WHO: Middle East respiratory syndrome coronavirus (MERS-CoV)
- WHO: Worldwide reduction in MERS cases and deaths since 2016