Innovative Dengue Vaccine Gains Another Authorization
A new vaccine targeting the fastest spreading mosquito-borne virus and one of the top ten threats to global health recently gained another authorization.
Takeda UK Ltd. announced on February 6, 2023, that the Medicines and Healthcare products Regulatory Agency (MHRA) had granted marketing authorization for Qdenga® for active immunization against dengue infection in individuals from four years of age.
Qdenga is currently the only MHRA-approved vaccine for preventing dengue fever, regardless of previous dengue exposure. Similar authorizations were issued in Europe and Indonesia in 2022.
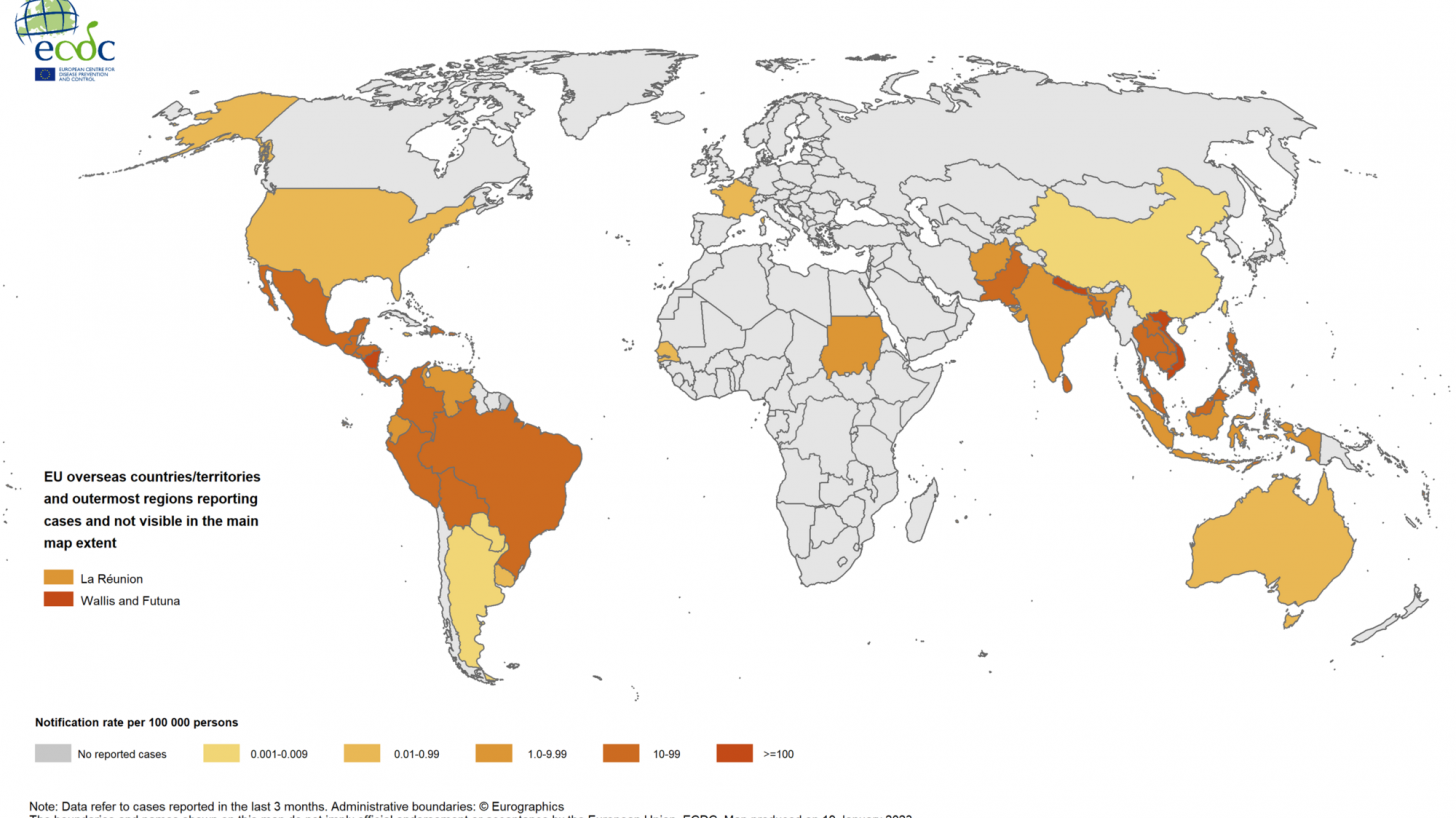
About half of the world's population is currently at risk of dengue, and it is now endemic in more than 125 countries, causing an estimated 500,000 hospitalizations annually.
Recently, south Florida and Puerto Rico confirmed locally-acquired dengue cases.
Furthermore, dengue is second only to Malaria as a diagnosed cause of fever among travelers returning to Europe.
The decision adopted by the MHRA follows positive results from the ongoing Phase 3, double-blind, randomized, placebo-controlled TIDES clinical trial.
Dr. George Kassianos, a specialist in immunizations, stated in a related press release, "Although the U.K. is not an endemic country, more than 800 UK holidaymakers had their trips ruined by dengue before the pandemic, with all reported cases imported from endemic territories."
"Most people with dengue will not have any symptoms, some will have a mild illness with flu-like symptoms, while rarely others may go on to have a more severe form of the infection."
"With this in mind, a pre-travel vaccination that provides an additional precautionary option to most travelers, in addition to following effective mosquito bite prevention, should be welcomed."
Dengue is predominantly spread to people through the bites of infected Aedes aegypti and Aedes albopictus mosquitoes.
The virus consists of four different viral serotypes (DENV-1, 2, 3, and 4), and infection with one serotype can offer long-term immunity against that serotype.
However, secondary infection with a different dengue serotype can lead to severe disease.
Simon Meadowcroft, Medical Director, Takeda UK & Ireland, commented... "The launch of Qdenga means that for the first time, those living in the U.K. and traveling to dengue-endemic countries can be vaccinated against infection."
Takeda UK Ltd. expects to make Qdenga® available in the U.K. in spring/summer 2023.
Qdenga is a dengue vaccine based on a live-attenuated dengue serotype 2 virus, which provides the genetic "backbone" for all four dengue virus serotypes and is designed to protect against any of these serotypes. The use of Qdenga should be in accordance with official recommendations, says Takeda.
Takeda announced in November 2022, that the U.S. Food and Drug Administration (FDA) had accepted and granted priority review of Qdenga's Biologics License Application.
In addition to Qdenga, the Dengvaxia® live attenuated tetravalent chimeric vaccine has been approved in various countries, including the U.S. FDA.
Furthermore, dengue vaccine candidates are conducting clinical studies in 2023.
Our Trust Standards: Medical Advisory Committee
























