Baylor Launches Clinical Trial of Imiquimod Topical Cream

Baylor College of Medicine in Houston has launched a Phase 1 clinical trial examining whether a topical cream can enhance the immune response conferred by a “pre-pandemic” influenza vaccine.
These investigators are evaluating whether imiquimod cream, which is commonly used to treat genital warts and certain skin cancers, can boost the body’s immune response to an H5N1 influenza vaccine.
Imiquimod cream activates the innate immune system — the body’s immediate defense against invading pathogens.
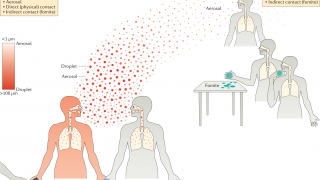
H5N1 is an avian influenza virus that causes severe respiratory illness in birds.
In rare circumstances, humans have contracted H5N1 influenza through direct or indirect contact with infected birds, such as poultry.
Infections in people can be serious and deadly — the World Health Organization reported 860 cases of H5N1 influenza, 454 of them fatal, from 2003 through July 20, 2018.
H5N1 influenza currently does not spread easily from human to human.
However, like all influenza viruses, the virus undergoes constant genetic changes, and it is possible that it may become more easily transmissible and cause a pandemic.
Baylor is one of the Vaccine and Treatment Evaluation Units (VTEUs) — a network of clinical research sites that can rapidly enroll large volunteer cohorts to evaluate experimental vaccines against infectious diseases.
Participants in the VTEU trial will receive an H5N1 vaccine manufactured by Sanofi Pasteur, based in Swiftwater, Pennsylvania that was designed for use in a potential pandemic.
The vaccine, developed with some NIAID support, is made from an inactivated, or “killed,” influenza virus. After the vaccine was approved by the Food and Drug Administration (FDA) in 2007, it was added to the National Pre-pandemic Influenza Vaccine Stockpile.
“NIAID is pleased to support a clinical trial evaluating an innovative way to boost immune responses to a pre-pandemic vaccine,” said NIAID Director Anthony S. Fauci, M.D.
“The Vaccine and Treatment Evaluation Units remain a crucial component of our pandemic influenza preparedness efforts.”
Investigators in Hong Kong recently conducted two clinical trials of imiquimod cream and seasonal inactivated influenza vaccine. One clinical trial enrolled elderly participants, and the other enrolled young adults.
The imiquimod cream was generally well-tolerated, and elderly and young participants who applied the cream before vaccination generated significantly more robust immune responses than those in control groups who did not receive imiquimod.
The immune-boosting properties of imiquimod could stretch the supply of H5N1 vaccines because fewer doses would be required to achieve sufficient immunity in recipients. This would allow more people to be vaccinated in an outbreak.
Hana M. El Sahly, M.D., associate professor, Department of Molecular Virology and Microbiology at Baylor College of Medicine, serves as the principal investigator for the clinical trial. All trial participants will receive two intradermal doses of the H5N1 vaccine, 21 days apart.
Participants will be randomly assigned to one of two treatment groups. One group will have Aldara, FDA-approved imiquimod cream manufactured by Valeant Pharmaceutical International, Inc., based in Laval, Canada applied to their upper arm before each vaccination.
The trial is double-blind, meaning the participants and the investigators do not know who received each type of topical cream. Study clinicians will monitor participants for any injection site reactions such as redness and swelling, or other reactions such as fever, headache or nausea.
After each injection, participants must refrain from washing their arm for four to six hours.
Participants will return to the clinic at regular intervals over the course of 7 months to have blood drawn so that investigators can test samples for an immune response.
After each vaccination, participants will take home a diary card to record any symptoms and medications taken at home. An independent data and safety monitoring committee will review participants’ clinical and safety data at specified time points and otherwise as needed.
The first clinical trial participant was vaccinated on June 19, 2018, and investigators expect to have early study results by the end of the year.
For more information, visit ClinicalTrials.gov.
The VTEUs are funded and managed by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets, and other NIAID-related materials are available on the NIAID website.
Our Trust Standards: Medical Advisory Committee