Coronavirus Convalescent Therapy Found Safe in Houston
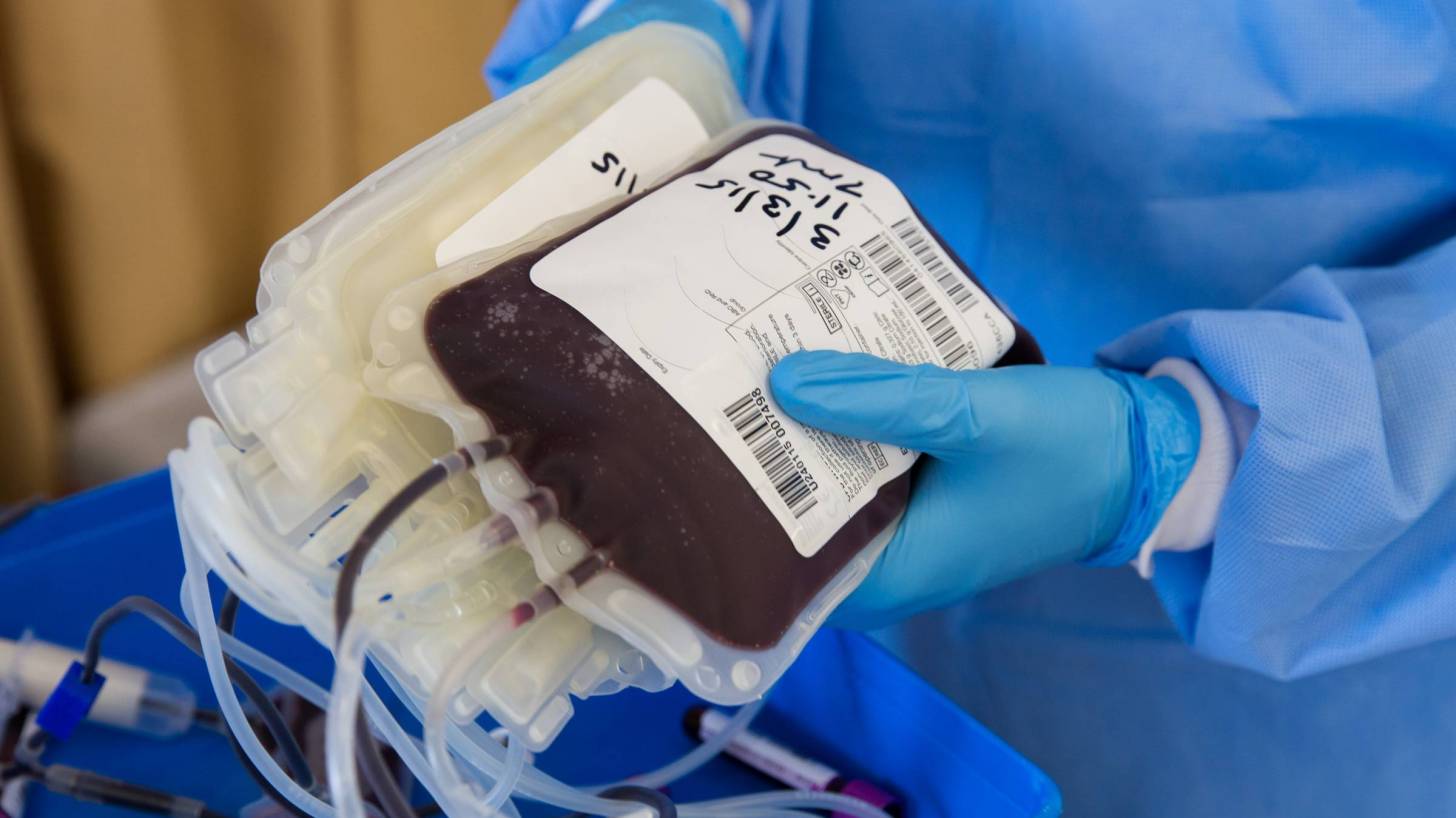
A non-peer-reviewed study published found coronavirus patients enrolled in an innovative clinical trial at Houston Methodist’s hospital, indicates that convalescent plasma treatment is a safe option for those hospitalized patients with severe COVID-19 disease.
Plasma therapy has been used since the Spanish flu in 1917–1918. And according to a recent study, the last reported utilization of plasma therapy was during the 2013–2015 Ebola virus outbreak in West Africa.
This type of convalescent plasma is an antibody-rich blood product made from blood donated by people who have recovered from the SARS-CoV-2 coronavirus.
Published on May 13, 2020, 25 patients with severe and/or life-threatening COVID-19 disease were enrolled at the Houston Methodist hospitals in Texas from March 28 to April 14, 2020.
These patients were transfused with convalescent plasma obtained from donors with confirmed SARS-CoV-2 infection and had been symptom-free for 14 days or more.
The primary study outcome was safety, and the secondary outcome was clinical status at day 14 post-transfusion.
At baseline, all of these patients were receiving supportive care, including anti-inflammatory, anti-viral treatments, and all patients were on oxygen support.
At day 7 post-transfusion with convalescent plasma, 9 patients had at least a 1-point improvement in clinical scale, and 7 of those were discharged.
By day 14 post-transfusion, 19 (76%) patients had at least a 1-point improvement in clinical status and 11 were discharged.
No adverse events as a result of plasma transfusion were observed during this study.
Furthermore, the whole-genome sequencing data did not identify a strain genotype-disease severity correlation.
These researchers concluded saying the ‘data indicate that the administration of convalescent plasma is a safe treatment option for those with severe COVID-19 disease.
Previously, the U.S. Food and Drug Administration (FDA) announced on April 3, 2020, it was taking the lead on a national effort to facilitate the development of, and access to, investigational therapies derived from human blood.
“This is an important area of research — the use of products made from a recovered patient’s blood to potentially treat COVID-19 in those affected by this illness,” said FDA Commissioner Stephen M. Hahn, M.D.
“The FDA had played a key role in organizing a partnership between industry, academic institutions, and government agencies to facilitate expanded access to convalescent plasma.”
These study authors have declared no competing interest. This study was supported by the National Institutes of Health grants, and the Fondren Foundation, Houston Methodist Hospital, and Research Institute.
Precision Vaccinations publishes coronavirus treatment news.
Our Trust Standards: Medical Advisory Committee

























