Hepatitis A Outbreaks Changed in 2016
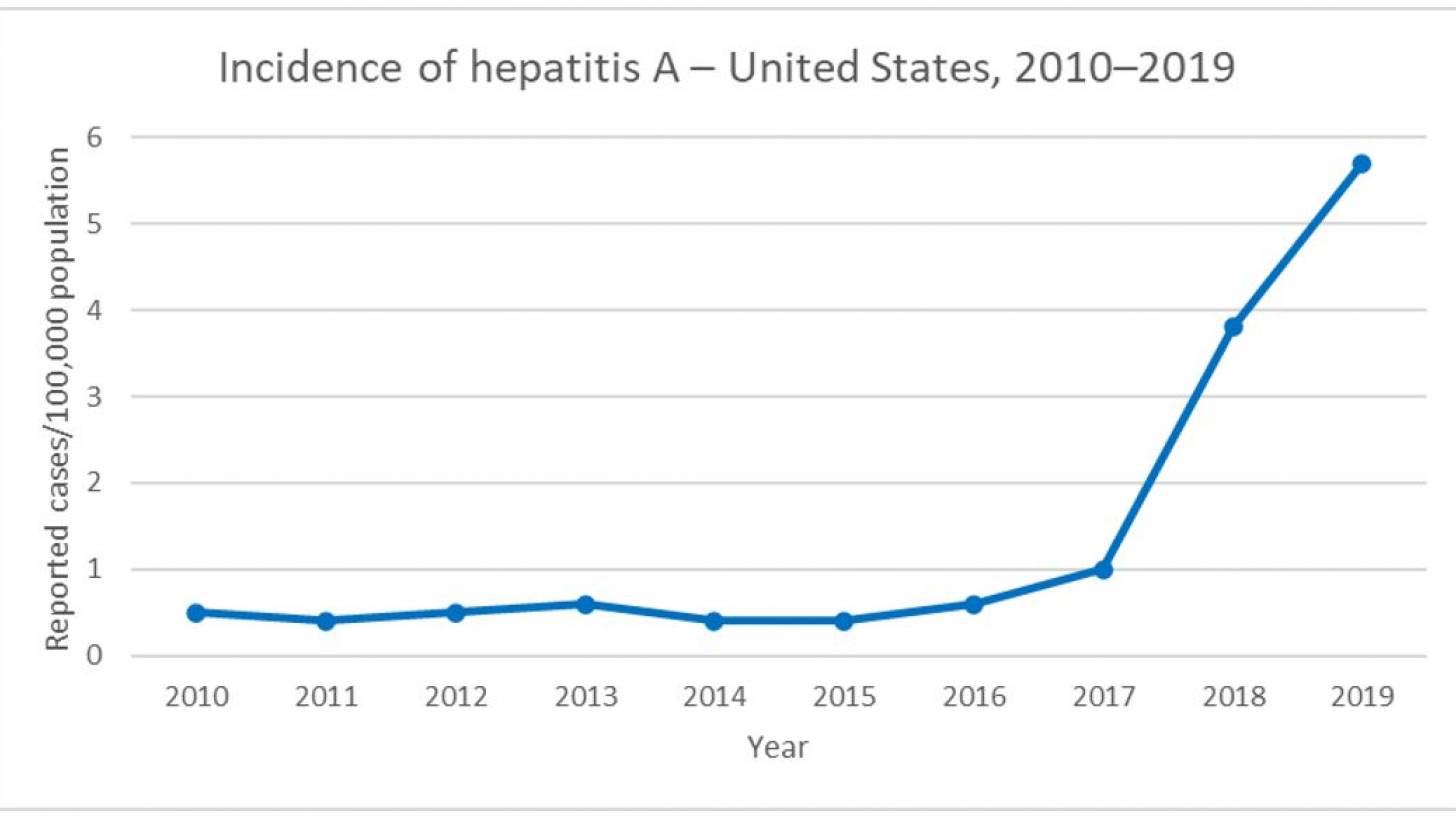
When most people hear about hepatitis A, they think about a virus associated with contaminated food and water. However, since 2016, hepatitis A has been more commonly spread from person to person, reported the U.S. Centers for Disease Control and Prevention (CDC).
The CDC's Division of Viral Hepatitis (DVH) reported that from August 2016 to October 7, 2020, 37 states reported hepatitis A outbreaks involving approximately 44,660 cases.
Because hepatitis A causes liver inflammation, people with other forms of viral hepatitis and anyone with underlying liver disease are at risk of more severe illness if infected.
Among cases with available information, 56% of persons reported drug use, 14% reported homelessness, and 61% were hospitalized, medium age of 38, and fewer than 1% of cases occurred among minors.
And among patients with available race data, about 81% occurred among White persons.
In total, 417 outbreak-associated fatalities have been reported.
In collaboration with state and local health departments, the CDC launched a large-scale, multidisciplinary response in 2017 to control the ongoing outbreaks.
The epidemiologic shifts identified during these outbreaks led to a 2019 recommendation by the CDC's Advisory Committee on Immunization Practices for vaccinating persons experiencing homelessness and reinforcement of existing vaccination recommendations for persons who use drugs.
As a result, substantial progress in preventing and controlling hepatitis A (HAV) has been made.
The number of outbreak-affected states has been reduced to 13.
According to the CDC, the current active leaders are Tennessee (3,157) and Indiana (2,683).
Health departments implemented nontraditional vaccination strategies to provide hepatitis A vaccination where needed.
These included holding satellite vaccination clinics and broadening the scope of healthcare professionals approved to administer vaccines.
To overcome barriers to vaccination, including mistrust, stigma, and vaccine hesitancy, health departments partnered with organizations that have long-standing trusted relationships with persons at risk for HAV infection, wrote the CDC.
Serologic testing is not required to administer a hepatitis A vaccine.
Despite long-standing vaccination recommendations, many adults at increased risk for HAV infection remain vulnerable to infection.
Given the hospitalization rate during these outbreaks and the high level of susceptibility to HAV infection, efforts are needed to improve awareness of and adherence to vaccination recommendations, concluded this CDC report.
Related Hepatitis A News
Countries Can Tackle Viral Hepatitis.
Hepatitis A, the Other Vaccine-Preventable Outbreak.
Most Hepatitis A Outbreaks Are Unannounced in the USA.
Will Hepatitis Be On the Menu in 2020?
The updated list of U.S. FDA-Approved hepatitis vaccines and vaccine candidates are published on this webpage.
Hepatitis A vaccines are generally available at clinics and pharmacies in the U.S.
Other hepatitis vaccine news is posted at PrecisionVaccinations.com/Hepatitis.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee
