Mosquirix Malaria Vaccine Effectiveness Measured By Antibody Quantity and Quality

A new study focused on the quantity and quality of antibodies recognizing the end region of the malaria parasite’s CSP protein is a good marker of protection by the Mosquirix (RTS,S/AS01E) vaccine, reported the Barcelona Institute for Global Health (ISGlobal).
Most vaccines contain inactivated pathogens or pathogen fragments against which the body produces protective antibodies.
This is not the case for Mosquirix, which has been the first malaria vaccine approved for large-scale pilot studies.
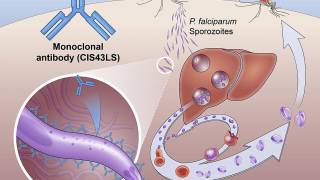
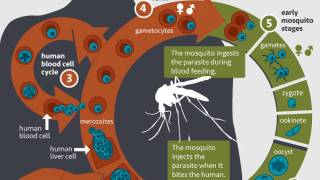
The Mosquirix vaccine contains a fragment of the Plasmodium falciparum CSP protein, that goes from the central part, rich in amino acid repeats (NANP region) to the end (called the C-terminal end).
These researchers measured not only the quantity of antibodies against the NANP and C-terminal regions but also their avidity.
Avidity indicates the strength with which antibodies bind to their ligand.
To do so, this study analyzed samples from over 1,000 infants, vaccinated or not during the Mosquirix phase 3 clinical trial in an area with low malaria transmission and 2 areas with high malaria transmission.
The results show for the first time that vaccination not only induces a strong increase in the number of antibodies against both CSP regions but also in their ‘avidity’.
This increase is stronger in children than in infants, which could explain why the former is better protected by this malaria vaccine.
Recent malaria news:
- Can Malaria Vaccine Mosquirix Save More Children?
- Severe Malaria Treatment Changed in the USA
- University of Chicago’s Malaria Vaccine Shows Greater Immune & Antibody Responses
“We see that, in terms of protection, the avidity of C-terminal antibodies is more important than the quantity, while for NANP antibodies quantity is more important than quality,” explains Carlota Dobaño, an Associate Research Professor at ISGlobal.
The results also indicate that when a child has already been exposed to malaria and therefore has anti-CSP antibodies, the protective effect of the vaccine is lower.
“This suggests that the vaccine will better protect children who have been less exposed to the parasite, for example, those living in low-transmission areas,” adds Dobaño.
The research team stresses the need to understand the mechanisms underlying the partial protection conferred by Mosquirix, in order to guide the design of new and better vaccines.
This is important news since malaria remains responsible for nearly 500,000 deaths annually, according to the Centers for Disease Control and Prevention (CDC).
About 1,700 cases of malaria are diagnosed in the United States annually, mostly in returning travelers.
The CDC publishes various travel alerts each year since millions of US residents travel to countries where malaria is present.
The study was supported by funds from PATH-Malaria Vaccine Initiative, National Institutes of Health- National Institute of Allergy and Infectious Diseases (USA, R01AI095789), the Ministry of Health in Ghana, the Ministerio de Economía y Competitividad (Instituto de Salud Carlos III, Spain, PI11/00423), EviMalaR and AGAUR-Catalonia (2014 SGR991).
The authors declare no competing interests.
Our Trust Standards: Medical Advisory Committee
- Protection by the Malaria Vaccine Is Not Only a Matter of Antibody Quantity but Also of Quality
- Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine
- RTS,S/AS01E Malaria Vaccine Induces Memory and Polyfunctional T Cell Responses in a Pediatric African Phase III Trial
- Malaria Vaccine Evaluation Programme (MVPE)