Clinical Trial Fails for Herpesvirus

Astellas Pharma Inc. announced its investigational DNA vaccine, ASP0113, being developed for cytomegalovirus (CMV)-seropositive hematopoietic stem cell transplant (HSCT) recipients, did not meet its primary or secondary endpoints in the Phase 3 HELIOS clinical trial.
Cytomegalovirus is a common virus that infects people of all ages. Over half of adults by age 40 have been infected with CMV, reports the Centers for Disease Control and Prevention (CDC).
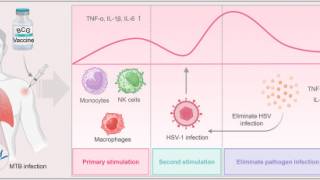
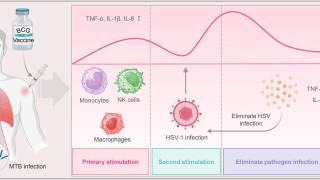
CMV is a herpesvirus, which is a group of viruses including herpes simplex virus types 1 and 2, varicella-zoster virus, and Epstein-Barr virus.
CMV spreads from person to person through body fluids, such as blood, saliva, urine, semen and breast milk.
Blood tests can be used to diagnose CMV infections in people who have symptoms.
Occasionally, CMV can cause a mononucleosis like illness or hepatitis.
Once CMV is in a person’s body, it stays there for life and can reactivate.
Most people infected with CMV show no signs or symptoms. However, CMV infection can cause serious health problems for people with weakened immune systems, as well as babies infected with the virus before they are born (congenital CMV).
Women can pass CMV to their baby during pregnancy. About one out of every 200 babies are born with congenital CMV infection.
However, only about one in five babies with congenital CMV infection will be sick from the virus or will have long-term health problems, says the CDC.
A healthy immune system typically protects an infected person against CMV disease but does not prevent or clear latent infection.
Those at greatest risk include HCT and solid-organ transplant recipients, as well as infants born to mothers who first become infected during pregnancy.
If you have CMV but are otherwise healthy, and you're experiencing mild, generalized illness, you could be in a reactivation period. If you know you were infected with CMV during your pregnancy, tell your baby's doctor.
The doctor should regularly assess your baby for hearing or vision problems, says the CDC.
This Phase 3 trial was designed to evaluate the efficacy of ASP0113 compared with placebo in CMV-seropositive recipients undergoing an allogeneic stem cell transplant.
The vaccine candidate ASP0113 is a bivalent DNA vaccine encoding CMV phosphoprotein 65 and glycoprotein B antigens for induction of both cellular and humoral immune responses, formulated with a proprietary poloxamer-based delivery system.
ASP0113 received Orphan Drug Designation in the United States and Europe.
For U.S. Media inquiries contact: Candace Johnson [email protected]
Our Trust Standards: Medical Advisory Committee