Expanded Malaria Treatment Option for Pregnant Women

Historically, malaria treatment options for pregnant women have been limited.
The United States has approximately 1,700 cases of imported malaria occurring each year, with approximately 37 percent of these cases occur in women, including 5–6 percent who are pregnant at the time they are infected.
When a woman is infected with malaria during pregnancy, they are at an increased risk for maternal and fetal complications, says the Centers for Disease Control and Prevention (CDC).
Treatment options for uncomplicated, chloroquine-resistant P. falciparum and P. vivax malaria infections in pregnant women are threatened by the spread of mefloquine resistance.
To address this need, the CDC’s Morbidity and Mortality Weekly Report published strong evidence of the safety and efficacy of using artemether-lumefantrine (AL, or Coartem) to treat malaria in the second and third trimesters of pregnancy.
The CDC report included various meta-analysis and randomized open-label, controlled trials that examined the efficacy of ACTs (artemisinin-based combination therapies) for uncomplicated P. falciparum in women during their second and third trimesters of pregnancy.
These researchers found cure rates greater than 94.9 percent, with ACTs performing equal to or better than quinine-based regimens.
On the basis of the evidence reviewed, the CDC recommends AL as an additional option for treatment of uncomplicated malaria in pregnant women in the United States during the second and third trimesters of pregnancy at the same doses recommended for nonpregnant women.
This CDC updated recommendation reflects current evidence and is consistent with existing malaria treatment guidelines.
"On the basis of the strength and quality of this evidence, the CDC recommends AL as an additional option for treatment of uncomplicated malaria in pregnant women in the United States during the second and third trimesters of pregnancy at the same doses recommended for nonpregnant women,” the study authors said.
Pregnant women in their first trimesters should still be treated with either mefloquine or quinine plus clindamycin, said these researchers.
Generally, the best way to prevent a disease is to be vaccinated. But, there is not a commercially available malaria vaccine available today.
***Schedule your appointment today ***
Recently, a malaria vaccine successfully completed Phase 3 testing, showed a 40 percent reduction in malaria.
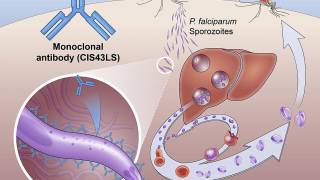
This vaccine, known as Mosquirix or RTS,S, acts against P. falciparum, the most deadly malaria parasite.
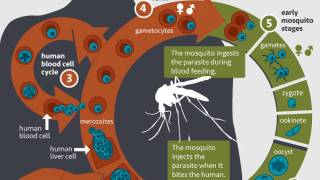
The Mosquirix vaccine aims to trigger the immune system to defend against the first stages when the Plasmodium falciparum malaria parasite enters the human host’s bloodstream through a mosquito bite and infects liver cells, reports GSK, the manufacturer of this vaccine.
To be effective, the vaccine has to be administered once a month for 3 months, then again 18 months later.
No conflicts of interest were reported by these researchers: Sarah-Blythe Ballard, MD, Ph.D.; Allison Salinger; MPHc; Paul M. Arguin, MD; Meghna Desai, Ph.D.; Kathrine R. Tan, MD.
The corresponding author: Sarah-Blythe Ballard, [email protected], 404-718-6711
Our Trust Standards: Medical Advisory Committee
- Updated CDC Recommendations for Using Artemether-Lumefantrine for the Treatment of Uncomplicated Malaria in Pregnant Women
- Clinical development of placental malaria vaccines and immunoassays harmonization
- Pre-clinical and clinical development of the first placental malaria vaccine.
- Clinical development of a VAR2CSA-based placental malaria vaccine PAMVAC
- Intermittent screening and treatment in pregnancy and the safety of ACTs in the first trimester
- Malaria Disease Found to be a Late Arrival