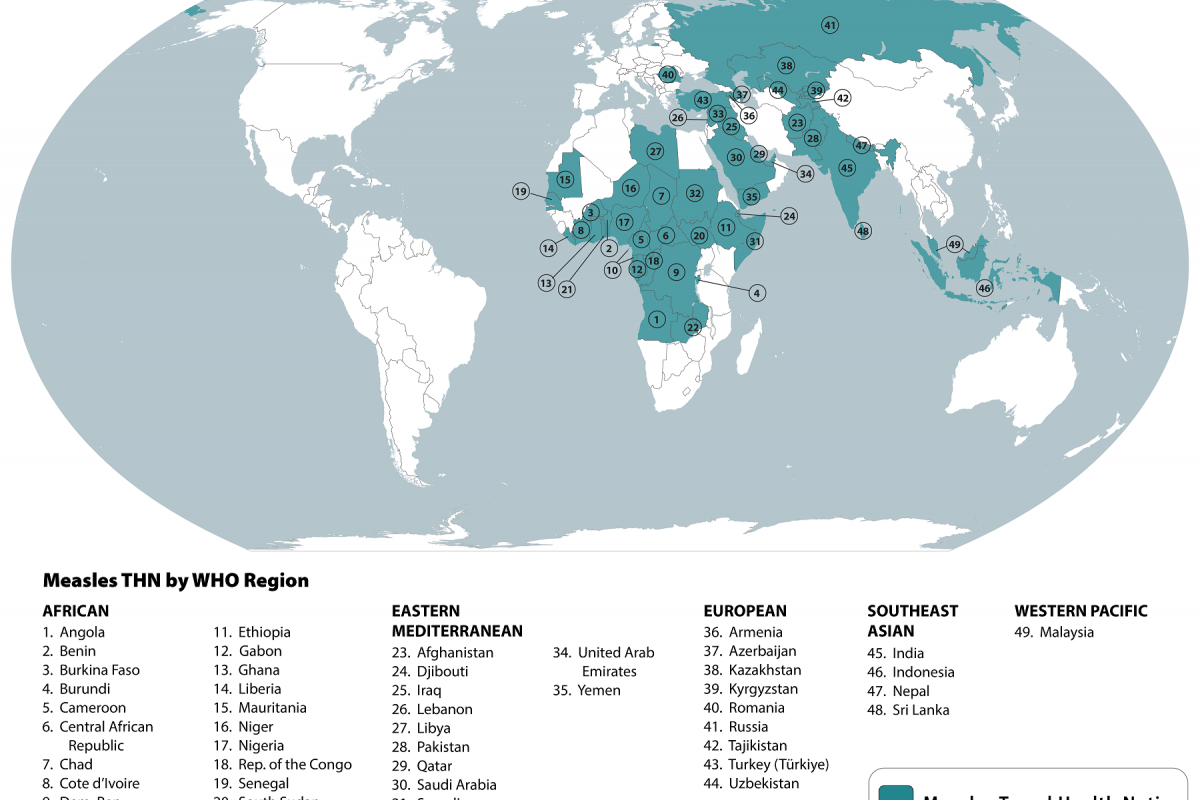
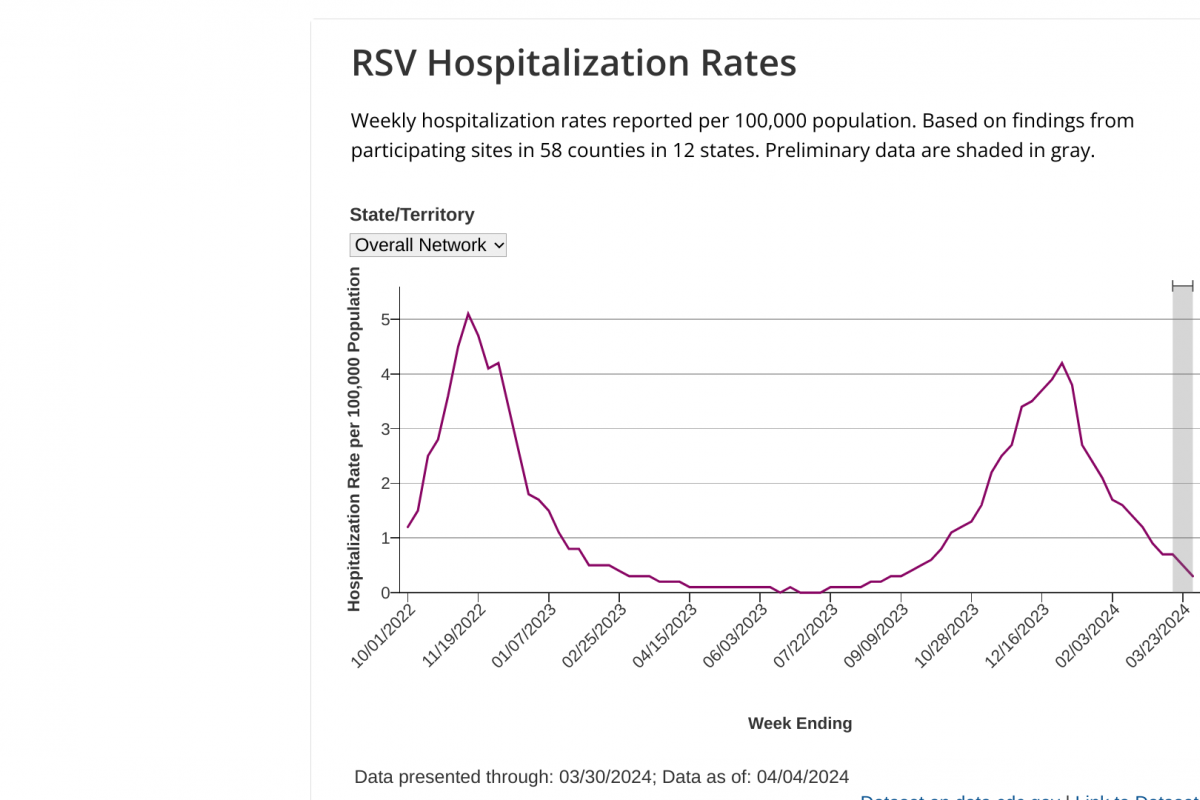
Nationally, weekly flu hospital admissions have been decreasing since January 2024, but small outbreaks continued to be reported in April 2024.
On April 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported outpatient respiratory illness declined and is below baseline for the first time since late October 2023, while HHS regions 1, 5, and 7 remain above their region-specific baselines.
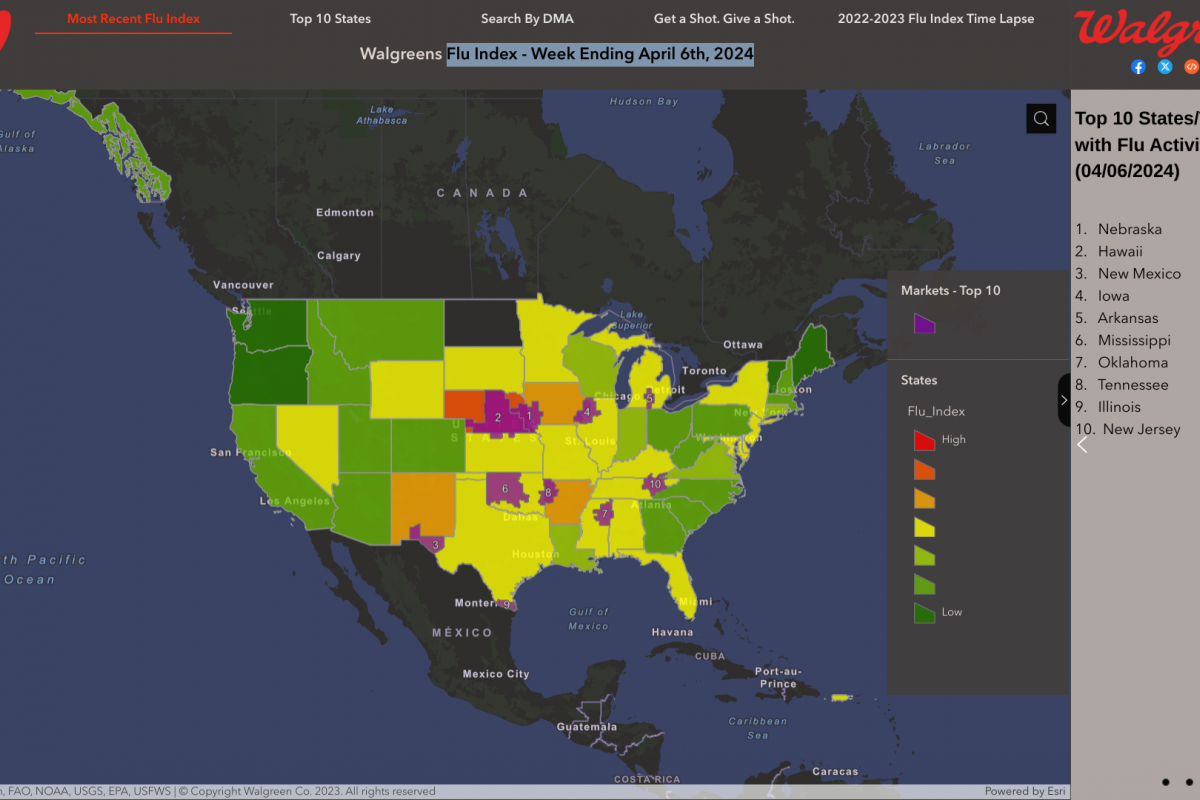
From a local perspective, the Walgreens Flu Index™ - Week Ending April 6, 2024, identified these ten Designated Market Areas with Flu Activity:
- Omaha, Neb.
- Lincoln & Hastings-Kearney, Neb.
- El Paso, Texas (Las Cruces, N.M.)
- Davenport, Iowa-Rock Island-Moline, Ill.
- Lansing, Mich.
- Oklahoma City, Okla.
- Columbus-Tupelo-West Point-Houston, Miss.
- Ft. Smith-Fayetteville-Springdale-Rogers, Ark.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Knoxville, Tenn.
The CDC's Weekly U.S. Influenza Surveillance Report also confirmed that five influenza-associated pediatric deaths occurring during the 2023-2024 season were reported to the CDC during Week 14, bringing the season total to 138 pediatric deaths.
Furthermore, the CDC recommends people speak with a healthcare provider about late-season flu shot options. Various egg, cell, and nasal-based influenza vaccines remain available at community pharmacies in the U.S.
Note: The Walgreens Flu Index displays information regarding flu activity compiled using retail prescription data for antiviral medications used to treat influenza.