Americans Will Have Access to Yellow Fever Vaccines

To meet the yellow fever vaccination needs, the Stamaril vaccine will continue to be available in the USA into mid-2021.
As announced by Sanofi Pasteur in France on December 23, 2020, the U.S. FDA has agreed to authorize the distribution of Stamaril through an Expanded Access Program (EAP) during the YF-VAX vaccine shortage. This EAP is forecast to be effective through mid-2021.
The EAP allows the importation and use of Stamaril until the YF-VAX vaccine production meets consumer demand. And should meet the yellow fever vaccination needs for U.S. military personnel and international travelers.
Stamaril is a live, attenuated yellow fever vaccine registered and currently distributed in over 70 countries but remains unlicensed in the USA.
‘We are making every effort to see that yellow fever vaccination continues in the U.S. during this YF-VAX vaccine supply disruption,’ stated a Sanofi press statement.
A U.S. EAP is similar to a clinical trial, and a limited number of clinical sites can participate in this program. More than 250 locations across the USA have been selected in this program.
YF-VAX vaccine is given to people nine months of age and older who are at increased risk for yellow fever to prevent them from getting the disease.
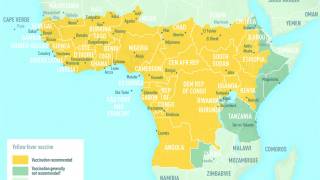
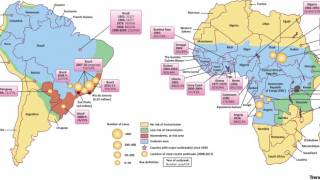
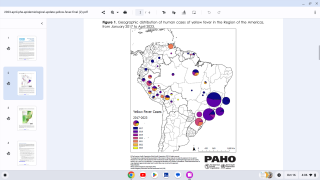
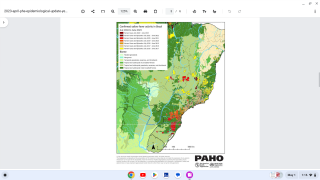
The yellow fever virus is found in tropical and subtropical areas of Africa and South America. It is an acute viral hemorrhagic fever caused by a virus transmitted through bites of infected mosquitoes.
Symptoms of yellow fever, such as fever, chills, headache, backache, and muscle aches, develop 3-6 days after infection. About 15% of people infected with the yellow fever virus will develop severe illness that can lead to liver disease, bleeding, shock, organ failure, yellowing skin, and sometimes is fatal.
People older than 60 years and people with weakened immune systems might be at higher risk of developing these side effects.
For international travelers, the US Centers for Disease Control and Prevention (CDC) publishes information on this webpage about which countries require yellow fever vaccination for entry and how countries the CDC recommends yellow fever vaccination.
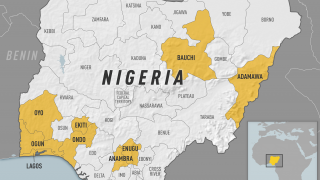
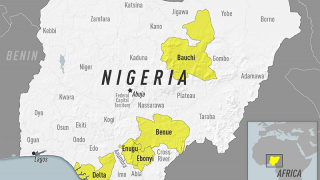
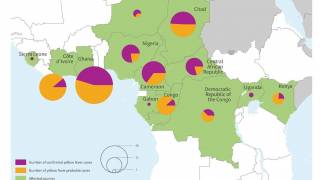
As a recent example, the CDC issued an Alert, Level 2, on November 30, 2020, regarding an outbreak of yellow fever in Nigeria. Therefore, travelers to Nigeria should prevent yellow fever by getting vaccinated at least ten days before travel and taking measures to avoid mosquito bites.
The Nigeria outbreak is currently in Bauchi, Benue, Delta, Ebonyi, and Enugu states. Unless vaccinated, travelers should not visit these areas, says the CDC.
International travelers should receive a yellow card called the International Certificate of Vaccination or Prophylaxis (ICVP) to prove that you have been immunized. Proof of vaccination is not valid until ten days after you get the vaccine, so plan to get the vaccine early if you need it.
Some countries require all travelers to show proof of yellow fever vaccination before they can enter the country. Other countries require proof of vaccination only if travelers have been in a risk area, so if you visit multiple countries, the order of travel may be necessary.
Unfortunately, fake ICVP certificates are found around the world.
“We estimate that around 80% of yellow fever travel cards in Zimbabwe are counterfeit,” said Dr. Mchechesi, a co-founder and head of innovation at Vaxiglobal, a travel health consultancy.
“It’s often impossible for busy border authorities to verify the names of doctors, and the supposed location where vaccinations occurred, by phone or email,” added Dr. Mchechesi.
International travelers should speak with a qualified yellow fever vaccine provider in advance of trips to high-risk countries, says the CDC.
PrecisionVaccinations publishes research-based news.
Our Trust Standards: Medical Advisory Committee