HPV16 Vaccine Candidate Proceeds with Initial Study

The start-up company PathoVax LLC announced that the U.S. Food and Drug Administration had concluded that a Phase 1 clinical trial for its monovalent component- HPV16 RG1-VLP vaccine RGVax may proceed.
This first-in-human, global multicenter Phase 1 clinical study seeks to demonstrate RGVax's safety and immunogenicity responses to HPV16 RG1-VLP in healthy volunteers.
RGVax is a chimeric HPV virus-like particle platform that displays 360 copies of the highly conserved, neutralizing HPV epitope (RG1).
The foundational technology is based on research conducted at the Johns Hopkins University and Medical University Vienna.
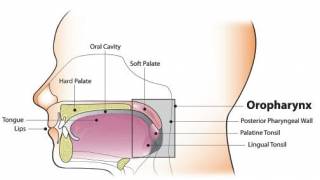
Unlike existing HPV vaccines, the RGVax technology and formulation have been shown to provide comprehensive protection against at least 18 high-risk human papillomavirus (HPV) types with immunogenicity lasting over a year without additional boosts in head-to-head studies with existing approved HPV vaccines.
"The world needs more, and particularly broad-based, HPV vaccines. We look forward to globally supporting these efforts in parallel and beyond this Phase 1, especially in Asia-Pacific and other developing countries, where there is a high burden of HPV diseases," said Dr. Kevin Koh, Chairman of PathoVax, in a press release on March 29, 2023.
A National Cancer Institute PREVENT contract funds the initiation of the Phase 1 study.
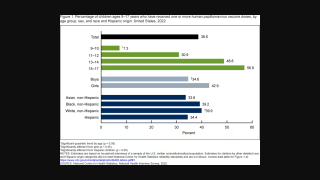
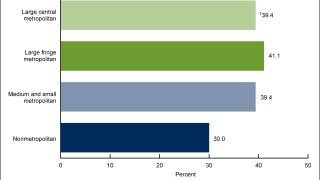
As of March 31, 2023, there are various approved HPV vaccines and several vaccine candidates in development.
Our Trust Standards: Medical Advisory Committee